Symptom clusters in COVID-19: A potential clinical prediction tool from the COVID Symptom Study app
- PMID: 33741586
- PMCID: PMC7978420
- DOI: 10.1126/sciadv.abd4177
Symptom clusters in COVID-19: A potential clinical prediction tool from the COVID Symptom Study app
Abstract
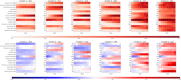
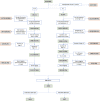
As no one symptom can predict disease severity or the need for dedicated medical support in coronavirus disease 2019 (COVID-19), we asked whether documenting symptom time series over the first few days informs outcome. Unsupervised time series clustering over symptom presentation was performed on data collected from a training dataset of completed cases enlisted early from the COVID Symptom Study Smartphone application, yielding six distinct symptom presentations. Clustering was validated on an independent replication dataset between 1 and 28 May 2020. Using the first 5 days of symptom logging, the ROC-AUC (receiver operating characteristic - area under the curve) of need for respiratory support was 78.8%, substantially outperforming personal characteristics alone (ROC-AUC 69.5%). Such an approach could be used to monitor at-risk patients and predict medical resource requirements days before they are required.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Emanuel E. J., Persad G., Upshur R., Thome B., Parker M., Glickman A., Zhang C., Boyle C., Smith M., Phillips J. P., Fair allocation of scarce medical resources in the time of Covid-19. N. Engl. J. Med. 382, 2049–2055 (2020). - PubMed
-
- Phua J., Weng L., Ling L., Egi M., Lim C. M., Divatia J. V., Shrestha B. R., Arabi Y. M., Ng J., Gomersall C. D., Nishimura M., Koh Y., Du B.; Asian Critical Care Clinical Trials Group , Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 8, 506–517 (2020). - PMC - PubMed
-
- Menni C., Valdes A. M., Freidin M. B., Sudre C. H., Nguyen L. H., Drew D. A., Ganesh S., Varsavsky T., Cardoso M. J., El-Sayed Moustafa J. S., Visconti A., Hysi P., Bowyer R. C. E., Mangino M., Falchi M., Wolf J., Ourselin S., Chan A. T., Steves C. J., Spector T. D., Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 26, 1037–1040 (2020). - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

