Neuronal Network Excitability in Alzheimer's Disease: The Puzzle of Similar versus Divergent Roles of Amyloid β and Tau
- PMID: 33741601
- PMCID: PMC8174042
- DOI: 10.1523/ENEURO.0418-20.2020
Neuronal Network Excitability in Alzheimer's Disease: The Puzzle of Similar versus Divergent Roles of Amyloid β and Tau
Abstract
Alzheimer's disease (AD) is the most frequent neurodegenerative disorder that commonly causes dementia in the elderly. Recent evidence indicates that network abnormalities, including hypersynchrony, altered oscillatory rhythmic activity, interneuron dysfunction, and synaptic depression, may be key mediators of cognitive decline in AD. In this review, we discuss characteristics of neuronal network excitability in AD, and the role of Aβ and tau in the induction of network hyperexcitability. Many patients harboring genetic mutations that lead to increased Aβ production suffer from seizures and epilepsy before the development of plaques. Similarly, pathologic accumulation of hyperphosphorylated tau has been associated with hyperexcitability in the hippocampus. We present common and divergent roles of tau and Aβ on neuronal hyperexcitability in AD, and hypotheses that could serve as a template for future experiments.
Keywords: amyloid β; neuronal excitability; seizures; tau.
Copyright © 2021 Kazim et al.
Figures


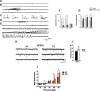
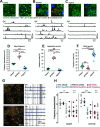

References
-
- Ahnaou A, Moechars D, Raeymaekers L, Biermans R, Manyakov NV, Bottelbergs A, Wintmolders C, Van Kolen K, Van De Casteele T, Kemp JA, Drinkenburg WH (2017) Emergence of early alterations in network oscillations and functional connectivity in a tau seeding mouse model of Alzheimer’s disease pathology. Sci Rep 7:14189. 10.1038/s41598-017-13839-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
