Uncovering Pathways to Personalized Therapies in Type 1 Diabetes
- PMID: 33741606
- PMCID: PMC7980192
- DOI: 10.2337/db20-1185
Uncovering Pathways to Personalized Therapies in Type 1 Diabetes
Abstract
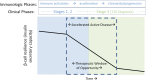
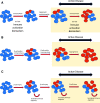
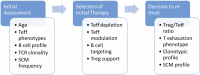
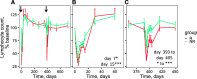
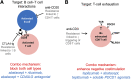
The goal of personalized medicine is to match the right drugs to the right patients at the right time. Personalized medicine has been most successful in cases where there is a clear genetic linkage between a disease and a therapy. This is not the case with type 1 diabetes (T1D), a genetically complex immune-mediated disease of β-cell destruction. Researchers over decades have traced the natural history of disease sufficiently to use autoantibodies as predictive biomarkers for disease risk and to conduct successful clinical trials of disease-modifying therapy. Recent studies, however, have highlighted heterogeneity associated with progression, with nonuniform rate of insulin loss and distinct features of the peri-diagnostic period. Likewise, there is heterogeneity in immune profiles and outcomes in response to therapy. Unexpectedly, from these studies demonstrating perplexing complexity in progression and response to therapy, new biomarker-based principles are emerging for how to achieve personalized therapies for T1D. These include therapy timed to periods of disease activity, use of patient stratification biomarkers to align therapeutic target with disease endotype, pharmacodynamic biomarkers to achieve personalized dosing and appropriate combination therapies, and efficacy biomarkers for "treat-to-target" strategies. These principles provide a template for application of personalized medicine to complex diseases.
© 2021 by the American Diabetes Association.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

