Production of l-Theanine by Escherichia coli in the Absence of Supplemental Ethylamine
- PMID: 33741612
- PMCID: PMC8208135
- DOI: 10.1128/AEM.00031-21
Production of l-Theanine by Escherichia coli in the Absence of Supplemental Ethylamine
Abstract
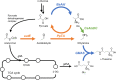
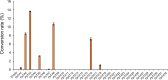
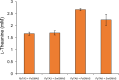
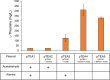
l-Theanine is a nonproteinogenic amino acid present almost exclusively in tea plants and is beneficial for human health. For industrial production, l-theanine is enzymatically or chemically synthesized from glutamine/glutamate (or a glutamine/glutamate derivative) and ethylamine. Ethylamine is extremely flammable and toxic, which complicates and increases the cost of operational procedures. To solve these problems, we developed an artificial biosynthetic pathway to produce l-theanine in the absence of supplemental ethylamine. For this purpose, we identified and selected a novel transaminase (NCBI:protein accession number AAN70747) from Pseudomonas putida KT2440, which catalyzes the transamination of acetaldehyde to produce ethylamine, as well as γ-glutamylmethylamide synthetase (NCBI:protein accession number AAY37316) from Pseudomonas syringae pv. syringae B728a, which catalyzes the condensation of l-glutamate and ethylamine to produce l-theanine. Expressing these genes in Escherichia coli W3110S3GK and enhancing the production capacity of acetaldehyde and l-alanine achieved successful production of l-theanine without ethylamine supplementation. Furthermore, the deletion of ggt, which encodes γ-glutamyltranspeptidase (EC 2.3.2.2), achieved large-scale production of l-theanine by attenuating its decomposition. We show that an alanine decarboxylase-utilizing pathway represents a promising route for the fermentative production of l-theanine. Our study reports efficient methods to produce l-theanine in the absence of supplemental ethylamine.IMPORTANCE l-Theanine is widely used in food additives and dietary supplements. Industrial production of l-theanine uses the toxic and highly flammable precursor ethylamine, raising production costs. In this study, we used Escherichia coli to engineer two biosynthetic pathways that produce l-theanine from glucose and ammonia in the absence of supplemental ethylamine. This study establishes a foundation for safely and economically producing l-theanine.
Keywords: Escherichia coli; alanine decarboxylase; l-theanine; theanine hydrolase; γ-glutamylmethylamide synthetase; ω-transaminase.
Copyright © 2021 American Society for Microbiology.
Figures


Similar articles
-
Efficient fermentative production of L-theanine by Corynebacterium glutamicum.Appl Microbiol Biotechnol. 2020 Jan;104(1):119-130. doi: 10.1007/s00253-019-10255-w. Epub 2019 Nov 27. Appl Microbiol Biotechnol. 2020. PMID: 31776607
-
Stable Isotope-Labeled Precursor Tracing Reveals that l-Alanine is Converted to l-Theanine via l-Glutamate not Ethylamine in Tea Plants In Vivo.J Agric Food Chem. 2021 Dec 22;69(50):15354-15361. doi: 10.1021/acs.jafc.1c06660. Epub 2021 Dec 14. J Agric Food Chem. 2021. PMID: 34904439
-
Application of Cell-Free Protein Synthesis System for the Biosynthesis of l-Theanine.ACS Synth Biol. 2021 Mar 19;10(3):620-631. doi: 10.1021/acssynbio.0c00618. Epub 2021 Mar 10. ACS Synth Biol. 2021. PMID: 33719397
-
A Comprehensive Review of l-Theanine Production in Escherichia coli: The Recent Progress, Metabolic Engineering Strategies, and Future Prospects.ACS Synth Biol. 2025 Jul 18;14(7):2433-2444. doi: 10.1021/acssynbio.5c00291. Epub 2025 Jul 4. ACS Synth Biol. 2025. PMID: 40613122 Review.
-
An overview of biological production of L-theanine.Biotechnol Adv. 2015 May-Aug;33(3-4):335-42. doi: 10.1016/j.biotechadv.2015.04.004. Epub 2015 Apr 12. Biotechnol Adv. 2015. PMID: 25871834 Review.
Cited by
-
Structure and evolution of alanine/serine decarboxylases and the engineering of theanine production.Elife. 2024 Sep 17;12:RP91046. doi: 10.7554/eLife.91046. Elife. 2024. PMID: 39287621 Free PMC article.
-
Enhanced production of D-pantothenic acid in Corynebacterium glutamicum using an efficient CRISPR-Cpf1 genome editing method.Microb Cell Fact. 2023 Jan 6;22(1):3. doi: 10.1186/s12934-023-02017-1. Microb Cell Fact. 2023. PMID: 36609377 Free PMC article.
-
Efficient cell factories for the production of N-methylated amino acids and for methanol-based amino acid production.Microb Biotechnol. 2022 Aug;15(8):2145-2159. doi: 10.1111/1751-7915.14067. Epub 2022 Apr 30. Microb Biotechnol. 2022. PMID: 35488805 Free PMC article. Review.
-
Promoting L-theanine accumulation in Camellia sinensis through irrigation with Pseudomonas knackmussii.Plant Cell Rep. 2025 Jul 14;44(8):175. doi: 10.1007/s00299-025-03556-0. Plant Cell Rep. 2025. PMID: 40658255
References
-
- Sakato Y. 1950. Studies on the chemical constituents of tea; III. A new amide theanine. Nippon Nōgeikagaku Kaishi 23:262–267. (In Japanese.) 10.1271/nogeikagaku1924.23.262. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

