A Novel Tyrosinase from Armillaria ostoyae with Comparable Monophenolase and Diphenolase Activities Suffers Substrate Inhibition
- PMID: 33741625
- PMCID: PMC8174770
- DOI: 10.1128/AEM.00275-21
A Novel Tyrosinase from Armillaria ostoyae with Comparable Monophenolase and Diphenolase Activities Suffers Substrate Inhibition
Abstract
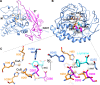
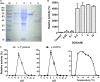
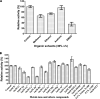
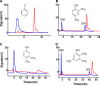
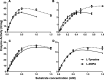
Tyrosinase is a bifunctional enzyme mediating the o-hydroxylation and two-electron oxidation of monophenols to o-quinones. The monophenolase activity of tyrosinase is much desired for the industrial synthesis of catechols. However, the generally low ratio of monophenolase/diphenolase activity of tyrosinase limited its utilization in the industry. In this study, a novel tyrosinase from Armillaria ostoyae strain C18/9 (AoTyr) was characterized, and the results showed that the enzyme has an optimal temperature of 25°C and an optimal pH of 6. The enzyme has comparable monophenolase and diphenolase activities and exhibits substrate inhibition in both of the activities. In silico analysis and mutagenesis experiments showed that residues 262 and 266 play important roles in modulating the substrate inhibition and enzymatic activities of AoTyr, and the replacement of D262 with asparagine significantly increased the monophenolase/diphenolase catalytic efficiencies (kcat/Km ratios) (1.63-fold) of the enzyme. The results from this study indicated that this novel tyrosinase could be a potential candidate for the industrial biosynthesis of catechols. IMPORTANCE Tyrosinase is able to oxidize various phenolic compounds, and its ability to convert monophenols into diphenols has caught great attention in the research field and industrial applications. However, the utilization of tyrosinase for the industrial synthesis of catechols has been limited due to the fact that the monophenolase activity of most of the known tyrosinases is much lower than the diphenolase activity. In the present study, a novel tyrosinase with comparable monophenolase and diphenolase activities was characterized. The enzyme exhibits substrate inhibition in both monophenolase and diphenolase activities. In silico analysis followed by mutagenesis experiments confirmed the important roles of residues 262 and 266 in the substrate inhibition and activity modulation of the enzyme, and the D262N variant showed an enhanced monophenolase/diphenolase catalytic efficiency ratio compared to the wild-type enzyme.
Keywords: Armillaria ostoyae; diphenolase; monophenolase; mutagenesis; substrate inhibition; tyrosinase.
Figures

Similar articles
-
Inhibitory effects of cupferron on the monophenolase and diphenolase activity of mushroom tyrosinase.Int J Biochem Cell Biol. 2003 Dec;35(12):1658-66. doi: 10.1016/s1357-2725(03)00006-2. Int J Biochem Cell Biol. 2003. PMID: 12962705
-
Kinetic characterization of substrate-analogous inhibitors of tyrosinase.IUBMB Life. 2015 Oct;67(10):757-67. doi: 10.1002/iub.1432. Epub 2015 Sep 24. IUBMB Life. 2015. PMID: 26399372
-
A novel high-resolution monophenolase/diphenolase/radical scavenging profiling for the rapid screening of natural whitening candidates from Peaonia lactiflora root and their mechanism study with molecular docking.J Ethnopharmacol. 2022 Jan 10;282:114607. doi: 10.1016/j.jep.2021.114607. Epub 2021 Sep 8. J Ethnopharmacol. 2022. PMID: 34506940
-
Considerations about the inhibition of monophenolase and diphenolase activities of tyrosinase. Characterization of the inhibitor concentration which generates 50 % of inhibition, type and inhibition constants. A review.Int J Biol Macromol. 2024 May;267(Pt 2):131513. doi: 10.1016/j.ijbiomac.2024.131513. Epub 2024 Apr 10. Int J Biol Macromol. 2024. PMID: 38608979 Review.
-
A review on spectrophotometric methods for measuring the monophenolase and diphenolase activities of tyrosinase.J Agric Food Chem. 2007 Nov 28;55(24):9739-49. doi: 10.1021/jf0712301. Epub 2007 Oct 25. J Agric Food Chem. 2007. PMID: 17958393 Review.
Cited by
-
Tyrosinases: a family of copper-containing metalloenzymes.ChemTexts. 2024;10(4):12. doi: 10.1007/s40828-024-00195-y. Epub 2024 Nov 30. ChemTexts. 2024. PMID: 39624788 Free PMC article.
-
Purification and Properties of Polyphenol Oxidase of Dried Volvariella bombycina.Biology (Basel). 2022 Dec 28;12(1):53. doi: 10.3390/biology12010053. Biology (Basel). 2022. PMID: 36671745 Free PMC article.
-
Contribution of the Tyrosinase (MoTyr) to Melanin Synthesis, Conidiogenesis, Appressorium Development, and Pathogenicity in Magnaporthe oryzae.J Fungi (Basel). 2023 Feb 28;9(3):311. doi: 10.3390/jof9030311. J Fungi (Basel). 2023. PMID: 36983479 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

