A Mycoplasma gallisepticum Glycerol ABC Transporter Involved in Pathogenicity
- PMID: 33741628
- PMCID: PMC8208142
- DOI: 10.1128/AEM.03112-20
A Mycoplasma gallisepticum Glycerol ABC Transporter Involved in Pathogenicity
Abstract
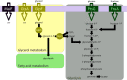
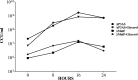
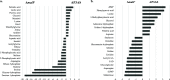
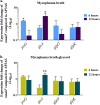
MalF has been shown to be required for virulence in the important avian pathogen Mycoplasma gallisepticum To characterize the function of MalF, predicted to be part of a putative ABC transporter, we compared metabolite profiles of a mutant with a transposon inserted in malF (MalF-deficient ST mutant 04-1; ΔmalF) with those of wild-type bacteria using gas chromatography-mass spectrometry and liquid chromatography-mass spectrometry. Of the substrates likely to be transported by an ABC transport system, glycerol was detected at significantly lower abundance in the ΔmalF mutant, compared to the wild type. Stable isotope labeling using [U-13C]glycerol and reverse transcription-quantitative PCR analysis indicated that MalF was responsible for the import of glycerol into M. gallisepticum and that, in the absence of MalF, the transcription of gtsA, which encodes a second transporter, GtsA, was upregulated, potentially to increase the import of glycerol-3-phosphate into the cell to compensate for the loss of MalF. The loss of MalF appeared to have a global effect on glycerol metabolism, suggesting that it may also play a regulatory role, and cellular morphology was also affected, indicating that the change to glycerol metabolism may have a broader effect on cellular organization. Overall, this study suggests that the reduced virulence of the ΔmalF mutant is due to perturbed glycerol uptake and metabolism and that the operon including malF should be reannotated as golABC to reflect its function in glycerol transport.IMPORTANCE Many mycoplasmas are pathogenic and cause disease in humans and animals. M. gallisepticum causes chronic respiratory disease in chickens and infectious sinusitis in turkeys, resulting in economic losses in poultry industries throughout the world. Expanding our knowledge about the pathogenesis of mycoplasma infections requires better understanding of the specific gene functions of these bacteria. In this study, we have characterized the metabolic function of a protein involved in the pathogenicity of M. gallisepticum, as well as its effect on expression of selected genes, cell phenotype, and H2O2 production. This study is a key step forward in elucidating why this protein plays a key role in virulence in chickens. This study also emphasizes the importance of functional characterization of mycoplasma proteins, using tools such as metabolomics, since prediction of function based on homology to other bacterial proteins is not always accurate.
Keywords: glycerol metabolism; metabolomics; mycoplasma.
Copyright © 2021 American Society for Microbiology.
Figures




Similar articles
-
MalF is essential for persistence of Mycoplasma gallisepticum in vivo.Microbiology (Reading). 2013 Jul;159(Pt 7):1459-1470. doi: 10.1099/mic.0.067553-0. Epub 2013 May 8. Microbiology (Reading). 2013. PMID: 23657682
-
Hydrogen peroxide production from glycerol metabolism is dispensable for virulence of Mycoplasma gallisepticum in the tracheas of chickens.Infect Immun. 2014 Dec;82(12):4915-20. doi: 10.1128/IAI.02208-14. Epub 2014 Aug 25. Infect Immun. 2014. PMID: 25156740 Free PMC article.
-
Metabolite profiling of Mycoplasma gallisepticum mutants, combined with bioinformatic analysis, can reveal the likely functions of virulence-associated genes.Vet Microbiol. 2018 Sep;223:160-167. doi: 10.1016/j.vetmic.2018.08.001. Epub 2018 Aug 2. Vet Microbiol. 2018. PMID: 30173742
-
Glycerol metabolism and its implication in virulence in Mycoplasma.FEMS Microbiol Rev. 2017 Sep 1;41(5):640-652. doi: 10.1093/femsre/fux033. FEMS Microbiol Rev. 2017. PMID: 28961963 Review.
-
Haemagglutinins of pathogenic avian mycoplasmas.Avian Pathol. 2002 Dec;31(6):535-47. doi: 10.1080/0307945021000024526. Avian Pathol. 2002. PMID: 12593736 Review.
Cited by
-
Unveiling genome plasticity and a novel phage in Mycoplasma felis: Genomic investigations of four feline isolates.Microb Genom. 2024 Mar;10(3):001227. doi: 10.1099/mgen.0.001227. Microb Genom. 2024. PMID: 38546735 Free PMC article.
-
A "plus one" strategy impacts replication of felid alphaherpesvirus 1, Mycoplasma and Chlamydia, and the metabolism of coinfected feline cells.mSystems. 2024 Oct 22;9(10):e0085224. doi: 10.1128/msystems.00852-24. Epub 2024 Sep 24. mSystems. 2024. PMID: 39315777 Free PMC article.
-
Mycoplasma synoviae lipid-associated membrane proteins identification and expression changes when exposed to chicken cells.Front Vet Sci. 2023 Nov 2;10:1249499. doi: 10.3389/fvets.2023.1249499. eCollection 2023. Front Vet Sci. 2023. PMID: 38026678 Free PMC article.
-
Genes required for survival and proliferation of Mycoplasma bovis in association with host cells.Appl Environ Microbiol. 2024 Jul 24;90(7):e0068724. doi: 10.1128/aem.00687-24. Epub 2024 Jun 12. Appl Environ Microbiol. 2024. PMID: 38864628 Free PMC article.
-
An unusual glycerol-3-phosphate dehydrogenase in Sulfolobus acidocaldarius elucidates the diversity of glycerol metabolism across Archaea.Commun Biol. 2025 Apr 1;8(1):539. doi: 10.1038/s42003-025-07953-9. Commun Biol. 2025. PMID: 40169898 Free PMC article.
References
-
- Kleven SH, Levisohn S. 1996. Mycoplasma infections of poultry, p 283–292. In Tully JG, Razin S (ed), Molecular and diagnostic procedures in mycoplasmology, 2nd ed. Academic Press, San Diego, CA.
-
- Masukagami Y, Nijagal B, Tseng C-W, Dayalan S, Tivendale KA, Markham PF, Browning GF, Sansom FM. 2018. Metabolite profiling of Mycoplasma gallisepticum mutants, combined with bioinformatic analysis, can reveal the likely functions of virulence-associated genes. Vet Microbiol 223:160–167. 10.1016/j.vetmic.2018.08.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

