Long-term Effects of Cholinesterase Inhibitors on Cognitive Decline and Mortality
- PMID: 33741639
- PMCID: PMC8166426
- DOI: 10.1212/WNL.0000000000011832
Long-term Effects of Cholinesterase Inhibitors on Cognitive Decline and Mortality
Abstract
Objective: To investigate whether cholinesterase inhibitors (ChEIs) are associated with slower cognitive decline in Alzheimer dementia and decreased risk of severe dementia or death.
Methods: Patients with Alzheimer dementia from the Swedish Dementia Registry starting on ChEIs within 3 months of the dementia diagnosis were included and compared to nontreated patients with Alzheimer dementia. In a propensity score-matched cohort, the association between ChEI use and cognitive trajectories assessed by Mini-Mental State Examination (MMSE) scores was examined with a mixed model, and severe dementia (MMSE score <10) or death as an outcome was assessed with Cox proportional hazards models.
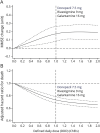
Results: The matched cohort included 11,652 ChEI users and 5,826 nonusers. During an average of 5 years of follow-up, 255 cases developed severe dementia, and 6,055 (35%) died. ChEI use was associated with higher MMSE score at each visit (0.13 MMSE points per year; 95% confidence interval [CI] 0.06-0.20). ChEI users had a 27% lower risk of death (0.73, 95% CI 0.69-0.77) compared with nonusers. Galantamine was associated with lower risk of death (0.71, 95% CI 0.65-0.76) and lower risk of severe dementia (0.69, 95% CI 0.47-1.00) and had the strongest effect on cognitive decline of all the ChEIs (0.18 MMSE points per year, 95% CI 0.07-0.28).
Conclusions: ChEIs are associated with cognitive benefits that are modest but persist over time and with reduced mortality risk, which could be explained partly by their cognitive effects. Galantamine was the only ChEI demonstrating a significant reduction in the risk of developing severe dementia.
Classification of evidence: This study provides Class III evidence that for patients with Alzheimer dementia ChEIs decrease long-term cognitive decline and risk of death and that galantamine decreases the risk of severe dementia.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
Comment in
-
Reader Response: Long-term Effects of Cholinesterase Inhibitors on Cognitive Decline and Mortality.Neurology. 2021 Nov 16;97(20):964-965. doi: 10.1212/WNL.0000000000012872. Neurology. 2021. PMID: 34782412 No abstract available.
-
Author Response: Long-term Effects of Cholinesterase Inhibitors on Cognitive Decline and Mortality.Neurology. 2021 Nov 16;97(20):965. doi: 10.1212/WNL.0000000000012874. Neurology. 2021. PMID: 34782413 No abstract available.
Similar articles
-
Long-term effects of cholinesterase inhibitors and memantine on cognitive decline, cardiovascular events, and mortality in dementia with Lewy bodies: An up to 10-year follow-up study.Alzheimers Dement. 2024 Oct;20(10):6740-6754. doi: 10.1002/alz.14118. Epub 2024 Aug 23. Alzheimers Dement. 2024. PMID: 39177108 Free PMC article.
-
Change in cognitive function according to cholinesterase inhibitor use and amyloid PET positivity in patients with mild cognitive impairment.Alzheimers Res Ther. 2021 Jan 5;13(1):10. doi: 10.1186/s13195-020-00749-5. Alzheimers Res Ther. 2021. PMID: 33402198 Free PMC article.
-
Cholinesterase Inhibitors May Not Benefit Mild Cognitive Impairment and Mild Alzheimer Disease Dementia.Alzheimer Dis Assoc Disord. 2019 Apr-Jun;33(2):87-94. doi: 10.1097/WAD.0000000000000291. Alzheimer Dis Assoc Disord. 2019. PMID: 30633043 Free PMC article.
-
Long-term cholinesterase inhibitor treatment of Alzheimer's disease.CNS Drugs. 2004;18(12):757-68. doi: 10.2165/00023210-200418120-00001. CNS Drugs. 2004. PMID: 15377166 Review.
-
Sex influences on cholinesterase inhibitor treatment in elderly individuals with Alzheimer's disease.Am J Geriatr Pharmacother. 2006 Sep;4(3):273-86. doi: 10.1016/j.amjopharm.2006.09.009. Am J Geriatr Pharmacother. 2006. PMID: 17062329 Review.
Cited by
-
Sex, environment, and death rate in a dementia cohort: a seven-years Bayesian survival analysis using medications data from a contaminated area in Italy.Front Public Health. 2024 Jun 11;12:1380609. doi: 10.3389/fpubh.2024.1380609. eCollection 2024. Front Public Health. 2024. PMID: 38952726 Free PMC article.
-
The Impact of Disease Registries on Advancing Knowledge and Understanding of Dementia Globally.Front Aging Neurosci. 2022 Feb 7;14:774005. doi: 10.3389/fnagi.2022.774005. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35197840 Free PMC article. Review.
-
The role of astrocytic α7 nicotinic acetylcholine receptors in Alzheimer disease.Nat Rev Neurol. 2023 May;19(5):278-288. doi: 10.1038/s41582-023-00792-4. Epub 2023 Mar 28. Nat Rev Neurol. 2023. PMID: 36977843 Review.
-
Combined use of Donepezil and Memantine increases the probability of five-year survival of Alzheimer's disease patients.Commun Med (Lond). 2024 May 23;4(1):99. doi: 10.1038/s43856-024-00527-6. Commun Med (Lond). 2024. PMID: 38783011 Free PMC article.
-
Use of anti-amyloid therapies for Alzheimer's disease in Brazil: a position paper from the Scientific Department of Cognitive Neurology and Aging of the Brazilian Academy of Neurology.Dement Neuropsychol. 2024 Nov 11;18:e2024C002. doi: 10.1590/1980-5764-DN-2024-C002. eCollection 2024. Dement Neuropsychol. 2024. PMID: 39534440 Free PMC article.
References
-
- Birks JS, Grimley Evans J. Rivastigmine for Alzheimer's disease. Cochrane database Syst Rev 2015:Cd001191. - PubMed
-
- Jelic V, Winblad B. Alzheimer disease. Donepezil and nursing home placement: benefits and costs. Nat Rev Neurol 2016;12:11–13. - PubMed
-
- Winblad B, Black SE, Homma A, et al. . Donepezil treatment in severe Alzheimer's disease: a pooled analysis of three clinical trials. Curr Med Res Opin 2009;25:2577–2587. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
