Noncanonical Roles of Caspase-4 and Caspase-5 in Heme-Driven IL-1β Release and Cell Death
- PMID: 33741688
- PMCID: PMC8026643
- DOI: 10.4049/jimmunol.2000226
Noncanonical Roles of Caspase-4 and Caspase-5 in Heme-Driven IL-1β Release and Cell Death
Abstract
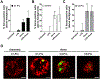
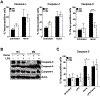
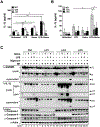
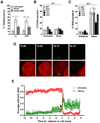
Excessive release of heme from RBCs is a key pathophysiological feature of several disease states, including bacterial sepsis, malaria, and sickle cell disease. This hemolysis results in an increased level of free heme that has been implicated in the inflammatory activation of monocytes, macrophages, and the endothelium. In this study, we show that extracellular heme engages the human inflammatory caspases, caspase-1, caspase-4, and caspase-5, resulting in the release of IL-1β. Heme-induced IL-1β release was further increased in macrophages from patients with sickle cell disease. In human primary macrophages, heme activated caspase-1 in an inflammasome-dependent manner, but heme-induced activation of caspase-4 and caspase-5 was independent of canonical inflammasomes. Furthermore, we show that both caspase-4 and caspase-5 are essential for heme-induced IL-1β release, whereas caspase-4 is the primary contributor to heme-induced cell death. Together, we have identified that extracellular heme is a damage-associated molecular pattern that can engage canonical and noncanonical inflammasome activation as a key mediator of inflammation in macrophages.
Copyright © 2021 by The American Association of Immunologists, Inc.
Conflict of interest statement
Conflict of Interest: JDB and GMV have research funding from CSL Behring and Mitobridge-Astellas. BAR is currently employed at Omniome Inc.
Figures


References
-
- van de Veerdonk FL, Netea MG, Dinarello CA, and Joosten LA. 2011. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol 32: 110–116. - PubMed
-
- Martin SJ 2016. Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPs of the immune system. FEBS J 283: 2599–2615. - PubMed
-
- Wagener FA, Eggert A, Boerman OC, Oyen WJ, Verhofstad A, Abraham NG, Adema G, van Kooyk Y, de Witte T, and Figdor CG. 2001. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood 98: 1802–1811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

