Conductance selectivity of Na+ across the K+ channel via Na+ trapped in a tortuous trajectory
- PMID: 33741736
- PMCID: PMC8000460
- DOI: 10.1073/pnas.2017168118
Conductance selectivity of Na+ across the K+ channel via Na+ trapped in a tortuous trajectory
Abstract
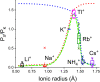
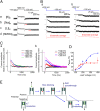
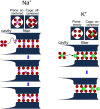
Ion selectivity of the potassium channel is crucial for regulating electrical activity in living cells; however, the mechanism underlying the potassium channel selectivity that favors large K+ over small Na+ remains unclear. Generally, Na+ is not completely excluded from permeation through potassium channels. Herein, the distinct nature of Na+ conduction through the prototypical KcsA potassium channel was examined. Single-channel current recordings revealed that, at a high Na+ concentration (200 mM), the channel was blocked by Na+, and this blocking was relieved at high membrane potentials, suggesting the passage of Na+ across the channel. At a 2,000 mM Na+ concentration, single-channel Na+ conductance was measured as one-eightieth of the K+ conductance, indicating that the selectivity filter allows substantial conduit of Na+ Molecular dynamics simulations revealed unprecedented atomic trajectories of Na+ permeation. In the selectivity filter having a series of carbonyl oxygen rings, a smaller Na+ was distributed off-center in eight carbonyl oxygen-coordinated sites as well as on-center in four carbonyl oxygen-coordinated sites. This amphipathic nature of Na+ coordination yielded a continuous but tortuous path along the filter. Trapping of Na+ in many deep free energy wells in the filter caused slow elution. Conversely, K+ is conducted via a straight path, and as the number of occupied K+ ions increased to three, the concerted conduction was accelerated dramatically, generating the conductance selectivity ratio of up to 80. The selectivity filter allows accommodation of different ion species, but the ion coordination and interactions between ions render contrast conduction rates, constituting the potassium channel conductance selectivity.
Keywords: KcsA channel; MD simulation; conductance ratio; lipid bilayer; single-channel current.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Hille B., Ion Channels of Excitable Membranes (Sinauer Associates Inc, Sunderland, ed. 3, 2001).
-
- Eisenman G., Horn R., Ionic selectivity revisited: The role of kinetic and equilibrium processes in ion permeation through channels. J. Membr. Biol. 76, 197–225 (1983). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

