Paradoxical androgen receptor regulation by small molecule enantiomers
- PMID: 33741738
- PMCID: PMC8000581
- DOI: 10.1073/pnas.2100918118
Paradoxical androgen receptor regulation by small molecule enantiomers
Abstract
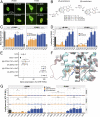
Small molecules that target the androgen receptor (AR) are the mainstay of therapy for lethal castration-resistant prostate cancer (CRPC), yet existing drugs lose their efficacy during continued treatment. This evolution of resistance is due to heterogenous mechanisms which include AR mutations causing the identical drug to activate instead of inhibit the receptor. Understanding in molecular detail the paradoxical phenomenon wherein an AR antagonist is transformed into an agonist by structural mutations in the target receptor is thus of paramount importance. Herein, we describe a reciprocal paradox: opposing antagonist and agonist AR regulation determined uniquely by enantiomeric forms of the same drug structure. The antiandrogen BMS-641988, which has (R)-chirality at C-5 encompasses a previously uncharacterized (S)-stereoisomer that is, surprisingly, a potent agonist of AR, as demonstrated by transcriptional assays supported by cell imaging studies. This duality was reproduced in a series of novel compounds derived from the BMS-641988 scaffold. Coupled with in silico modeling studies, the results inform an AR model that explains the switch from potent antagonist to high-affinity agonist in terms of C-5 substituent steric interactions with helix 12 of the ligand binding site. They imply strategies to overcome AR drug resistance and demonstrate that insufficient enantiopurity in this class of AR antagonist can confound efforts to correlate structure with function.
Keywords: androgen receptor; cancer; chirality; drug testing.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

