Efficacy and Safety of Everolimus With Reduced Tacrolimus in Liver Transplant Recipients: 24-month Results From the Pooled Analysis of 2 Randomized Controlled Trials
- PMID: 33741847
- PMCID: PMC8221719
- DOI: 10.1097/TP.0000000000003394
Efficacy and Safety of Everolimus With Reduced Tacrolimus in Liver Transplant Recipients: 24-month Results From the Pooled Analysis of 2 Randomized Controlled Trials
Abstract
Background and methods: Data from 2 randomized liver transplant trials (N = 772; H2304 [deceased donor, n = 488], H2307 [living donor, n = 284]) were pooled to further evaluate the efficacy and safety of everolimus with reduced tacrolimus (EVR + rTAC) versus standard tacrolimus (sTAC) regimen at month 24.
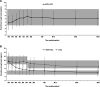
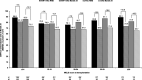
Results: EVR + rTAC was comparable to sTAC for composite efficacy failure of treated biopsy-proven acute rejection, graft loss, or death (9.8% versus 10.8%; difference, -1.0%; 95% confidence interval, -5.4 to 3.4; P = 0.641) at month 24. EVR + rTAC was superior to sTAC for the mean change in estimated glomerular filtration rate (eGFR) from randomization to month 24 (-8.37 versus -13.40 mL/min/1.73 m2; P = 0.001). A subanalysis of renal function by chronic kidney disease (CKD) stage at randomization showed significantly lower decline in eGFR from randomization to month 24 for patients with CKD stage 1/2 (eGFR ≥ 60 mL/min/1.73 m2) in EVR + rTAC group versus sTAC (-12.82 versus -17.67 mL/min/1.73 m2, P = 0.009). In patients transplanted for hepatocellular carcinoma (HCC) beyond Milan criteria, HCC recurrence was numerically lower although not statistically significant with EVR + rTAC versus sTAC group (5.9% [1 of 17] versus 23.1% [6 of 26], P = 0.215), while comparable in patients within Milan criteria (2.9% [3 of 102] versus 2.1% [2 of 96], P = 1.000), irrespective of pretransplant alpha-fetoprotein levels.
Conclusions: EVR + rTAC versus sTAC showed comparable efficacy and safety with significantly better renal function, particularly in patients with normal/mildly decreased renal function (CKD stage 1/2) at randomization and a trend toward lower HCC recurrence in patients transplanted with HCC beyond Milan at month 24. Further long-term data would be required to confirm these results.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
F.S. has received speaker honoraria and/or research grants from Novartis, Astellas, Chiesi, Genzyme, Gilead, AbbVie, Merck, Sharp & Dohme, Pfizer, Gambro and Baxter. P.D.S. is an advisory board member and has received speaker’s fees from Novartis. F.N. has received research grants from Astellas, Ipsen, and MSD and has served as consultant for Gilead, AbbVie, Promethera Biosciences, Durect, Ferring, Gore, Cook Medical, TwinPharma, Ipsen, and Intercept. L.F. has received speaker honoraria (Novartis Pharma, Astellas Pharma), has served as a member of Data and Safety Monitoring Board (Novartis Pharma), and is involved in clinical trials sponsored by Astellas Pharma, Chiesi, Novartis Pharma, and Quark Pharmaceuticals. D.J.J. has received speaker honoraria from Novartis. J.F. has received educational grant from Sanofi and has served as a member of Drug Safety Monitoring Board. M.M., C.S., and S.K. are employees of Novartis. B.R. is an ex-employee of Novartis. G.L. has served as consultant for Astellas, Novartis, Therapure Pharm Inc, Veritas Therapeutics and is a member of scientific advisory board of Singapore MRC. The other authors declare no conflicts of interest.
Figures



Comment in
-
What Is the Impact of Mammalian Target of Rapamycin Inhibitors on Hepatocellular Carcinoma Recurrence After Liver Transplantation.Transplantation. 2022 Mar 1;106(3):e189. doi: 10.1097/TP.0000000000003980. Transplantation. 2022. PMID: 35192584 No abstract available.
References
-
- Kim WR, Lake JR, Smith JM, et al. . OPTN/SRTR 2016 annual data report: liver. Am J Transplant. 2018; 18Suppl 1172–253. doi: 10.1111/ajt.14559 - PubMed
-
- Adam R, Karam V, Cailliez V, et al. ; all the other 126 contributing centers (www.eltr.org) and the European Liver and Intestine Transplant Association (ELITA). 2018 Annual Report of the European Liver Transplant Registry (ELTR)—50-year evolution of liver transplantation. Transpl Int. 2018; 31121293–1317. doi: 10.1111/tri.13358 - PubMed
-
- Chen CL, Kabiling CS, Concejero AM. Why does living donor liver transplantation flourish in Asia? Nat Rev Gastroenterol Hepatol. 2013; 1012746–751. doi: 10.1038/nrgastro.2013.194 - PubMed
-
- Trotter JF. Liver transplantation around the world. Curr Opin Organ Transplant. 2017; 222123–127. doi: 10.1097/MOT.0000000000000392 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

