An endothelial activin A-bone morphogenetic protein receptor type 2 link is overdriven in pulmonary hypertension
- PMID: 33741934
- PMCID: PMC7979873
- DOI: 10.1038/s41467-021-21961-3
An endothelial activin A-bone morphogenetic protein receptor type 2 link is overdriven in pulmonary hypertension
Abstract
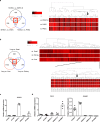
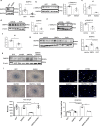
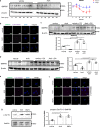
Pulmonary arterial hypertension is a progressive fatal disease that is characterized by pathological pulmonary artery remodeling, in which endothelial cell dysfunction is critically involved. We herein describe a previously unknown role of endothelial angiocrine in pulmonary hypertension. By searching for genes highly expressed in lung microvascular endothelial cells, we identify inhibin-β-A as an angiocrine factor produced by pulmonary capillaries. We find that excess production of inhibin-β-A by endothelial cells impairs the endothelial function in an autocrine manner by functioning as activin-A. Mechanistically, activin-A induces bone morphogenetic protein receptor type 2 internalization and targeting to lysosomes for degradation, resulting in the signal deficiency in endothelial cells. Of note, endothelial cells isolated from the lung of patients with idiopathic pulmonary arterial hypertension show higher inhibin-β-A expression and produce more activin-A compared to endothelial cells isolated from the lung of normal control subjects. When endothelial activin-A-bone morphogenetic protein receptor type 2 link is overdriven in mice, hypoxia-induced pulmonary hypertension was exacerbated, whereas conditional knockout of inhibin-β-A in endothelial cells prevents the progression of pulmonary hypertension. These data collectively indicate a critical role for the dysregulated endothelial activin-A-bone morphogenetic protein receptor type 2 link in the progression of pulmonary hypertension, and thus endothelial inhibin-β-A/activin-A might be a potential pharmacotherapeutic target for the treatment of pulmonary arterial hypertension.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Galie N, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the Internat. Eur. Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

