trans-Translation inhibitors bind to a novel site on the ribosome and clear Neisseria gonorrhoeae in vivo
- PMID: 33741965
- PMCID: PMC7979765
- DOI: 10.1038/s41467-021-22012-7
trans-Translation inhibitors bind to a novel site on the ribosome and clear Neisseria gonorrhoeae in vivo
Abstract
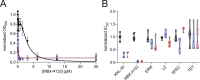
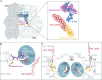
Bacterial ribosome rescue pathways that remove ribosomes stalled on mRNAs during translation have been proposed as novel antibiotic targets because they are essential in bacteria and are not conserved in humans. We previously reported the discovery of a family of acylaminooxadiazoles that selectively inhibit trans-translation, the main ribosome rescue pathway in bacteria. Here, we report optimization of the pharmacokinetic and antibiotic properties of the acylaminooxadiazoles, producing MBX-4132, which clears multiple-drug resistant Neisseria gonorrhoeae infection in mice after a single oral dose. Single particle cryogenic-EM studies of non-stop ribosomes show that acylaminooxadiazoles bind to a unique site near the peptidyl-transfer center and significantly alter the conformation of ribosomal protein bL27, suggesting a novel mechanism for specific inhibition of trans-translation by these molecules. These results show that trans-translation is a viable therapeutic target and reveal a new conformation within the bacterial ribosome that may be critical for ribosome rescue pathways.
Conflict of interest statement
Authors Z.D.A., M.C.T., J.S.B., S.C.C., S.M.K., L.R.M., M.M.B., T.J.O., and T.L.B. are employees of Microbiotix, Inc. Authors Z.D.A., S.M.K., M.C.T., J.S.B., S.C.C., M.M.B., T.J.O., and K.C.K. are inventors on a provisional patent covering molecules MBX-4346, MBX-4699, MBX-4700, MBX-4697, and MBX-4132.
Figures


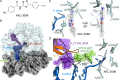
References
-
- Interagency Coordination Group on Antimicrobial Resistance. WHO | No Time to Wait: Securing the future from drug-resistant infections. http://www.who.int/antimicrobial-resistance/interagency-coordination-gro... (2019).
-
- U. S. Department of Health and Human Services CDC. Cdc. Antibiot. Resistance Threats U. S. 2019;2019:148.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

