Ad26.COV2.S protects Syrian hamsters against G614 spike variant SARS-CoV-2 and does not enhance respiratory disease
- PMID: 33741993
- PMCID: PMC7979827
- DOI: 10.1038/s41541-021-00301-y
Ad26.COV2.S protects Syrian hamsters against G614 spike variant SARS-CoV-2 and does not enhance respiratory disease
Abstract
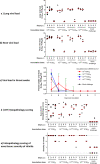
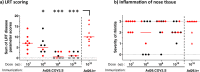
Previously we have shown that a single dose of recombinant adenovirus serotype 26 (Ad26) vaccine expressing a prefusion stabilized SARS-CoV-2 spike antigen (Ad26.COV2.S) is immunogenic and provides protection in Syrian hamster and non-human primate SARS-CoV-2 infection models. Here, we investigated the immunogenicity, protective efficacy, and potential for vaccine-associated enhanced respiratory disease (VAERD) mediated by Ad26.COV2.S in a moderate disease Syrian hamster challenge model, using the currently most prevalent G614 spike SARS-CoV-2 variant. Vaccine doses of 1 × 109 and 1 × 1010 VP elicited substantial neutralizing antibodies titers and completely protected over 80% of SARS-CoV-2 inoculated Syrian hamsters from lung infection and pneumonia but not upper respiratory tract infection. A second vaccine dose further increased neutralizing antibody titers that was associated with decreased infectious viral load in the upper respiratory tract after SARS-CoV-2 challenge. Suboptimal non-protective immune responses elicited by low-dose A26.COV2.S vaccination did not exacerbate respiratory disease in SARS-CoV-2-inoculated Syrian hamsters with breakthrough infection. In addition, dosing down the vaccine allowed to establish that binding and neutralizing antibody titers correlate with lower respiratory tract protection probability. Overall, these preclinical data confirm efficacy of a one-dose vaccine regimen with Ad26.COV2.S in this G614 spike SARS-CoV-2 virus variant Syrian hamster model, show the added benefit of a second vaccine dose, and demonstrate that there are no signs of VAERD under conditions of suboptimal immunity.
Conflict of interest statement
J.E.M.vdL., S.K.R.H., A.V., L.D., E.vH., J.V., Y.C., M.R.M.B., K.F.-dB., A.I.G., M.vH., J.T.B.M.T., J.S., L.M., L.vdF., L.R., J.P.M.L., D.H.B., H.S., R.C.Z., and F.W. are employees of Janssen Vaccines & Prevention. All authors may own stock or stock options in Johnson & Johnson, the parent company of Janssen Vaccines & Prevention.
Figures




References
-
- John Hopkins University Coronavirus Resource Centre. https://coronavirus.jhu.edu/ (2020).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

