Amorphous cellulose nanofiber supercapacitors
- PMID: 33742038
- PMCID: PMC7979786
- DOI: 10.1038/s41598-021-85901-3
Amorphous cellulose nanofiber supercapacitors
Abstract
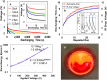
Despite the intense interest in cellulose nanofibers (CNFs) for biomedical and engineering applications, no research findings about the electrical energy storage of CNF have been reported yet. Here, we present the first electroadsorption effects of an amorphous cellulose nanofiber (ACF) supercapacitor, which can store a large amount of electricity (221 mJm-2, 13.1 Wkg-1). The electric storage can be attributed to the entirely enhanced electroadsorption owing to a quantum-size effect by convexity of 17.9 nm, an offset effect caused by positive polar C6=O6 radicles, and an electrostatic effect by appearance of the localised electrons near the Na ions. The supercapacitor also captures both positive and negative electricity from the atmosphere and in vacuum. The supercapacitor could illuminate a red LED for 1 s after charging it with 2 mA at 10 V. Further gains might be attained by integrating CNF specimens with a nano-electromechanical system (NEMS).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Abdel-karim AM, Salama AH, Hassan ML. Electrical conductivity and dielectric properties of nanofibrillated cellulose thin films from bagasse. J. Phys. Org. Chem. 2018;31:e3851. doi: 10.1002/poc.3851. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

