Immune microenvironment characterisation and dynamics during anti-HER2-based neoadjuvant treatment in HER2-positive breast cancer
- PMID: 33742063
- PMCID: PMC7979716
- DOI: 10.1038/s41698-021-00163-6
Immune microenvironment characterisation and dynamics during anti-HER2-based neoadjuvant treatment in HER2-positive breast cancer
Abstract
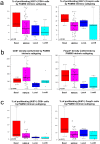
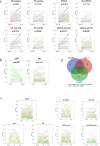
Despite their recognised role in HER2-positive (HER2+) breast cancer (BC), the composition, localisation and functional orientation of immune cells within tumour microenvironment, as well as its dynamics during anti-HER2 treatment, is largely unknown. We here investigate changes in tumour-immune contexture, as assessed by stromal tumour-infiltrating lymphocytes (sTILs) and by multiplexed spatial cellular phenotyping, during treatment with lapatinib-trastuzumab in HER2+ BC patients (PAMELA trial). Moreover, we evaluate the relationship of tumour-immune contexture with hormone receptor status, intrinsic subtype and immune-related gene expression. sTIL levels increase after 2 weeks of HER2 blockade in HR-negative disease and HER2-enriched subtype. This is linked to a concomitant increase in cell density of all four immune subpopulations (CD3+, CD4+, CD8+, Foxp3+). Moreover, immune contexture analysis showed that immune cells spatially interacting with tumour cells have the strongest association with response to anti-HER2 treatment. Subsequently, sTILs consistently decrease at the surgery in patients achieving pathologic complete response, whereas most residual tumours at surgery remain inflamed, possibly reflecting a progressive loss of function of T cells. Understanding the features of the resulting tumour immunosuppressive microenvironment has crucial implications for the design of new strategies to de-escalate or escalate systemic therapy in early-stage HER2+ BC.
Conflict of interest statement
A.P. reports consulting fees from Nanostring Technologies, Roche, Pfizer, Oncolytics Biotech, Puma, Lilly, AstraZeneca, Seattle Genetics, Novartis, Amgen, BMS, MSD and Daiichi Sankyo outside the submitted work. P.N. reports consulting fees of advisory boards from Bayer, MSD and Novartis and speaker fees from MSD and Novartis. All other authors declared no competing interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

