Does a high Mandard score really define a poor response to chemotherapy in oesophageal adenocarcinoma?
- PMID: 33742143
- PMCID: PMC8110771
- DOI: 10.1038/s41416-021-01290-4
Does a high Mandard score really define a poor response to chemotherapy in oesophageal adenocarcinoma?
Abstract
Background: A high Mandard score implies a non-response to chemotherapy in oesophageal adenocarcinoma. However, some patients exhibit tumour volume reduction and a nodal response despite a high score. This study examines survival and recurrence patterns in these patients.
Methods: Clinicopathological factors were analysed using multivariable Cox regression assessing time to death and recurrence. Computed tomography-estimated tumour volume change was examined in a subgroup of consecutive patients.
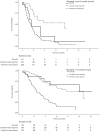
Results: Five hundred and fifty-five patients were included. Median survival was 55 months (Mandard 1-3) and 21 months (Mandard 4 and 5). In the Mandard 4 and 5 group (332 patients), comparison between complete nodal responders and persistent nodal disease showed improved survival (90 vs 18 months), recurrence rates (locoregional 14.75 vs 28.74%, systemic 24.59 vs 48.42%) and circumferential resection margin positivity (22.95 vs 68.11%). Complete nodal response independently predicted improved survival (hazard ratio 0.34 (0.16-0.74). Post-chemotherapy tumour volume reduction was greater in patients with a complete nodal response (-16.3 vs -7.7 cm3, p = 0.033) with no significant difference between Mandard groups.
Conclusion: Patients with a complete nodal response to chemotherapy have significantly improved outcomes despite a poor Mandard score. High Mandard score does not correspond with a non-response to chemotherapy in all cases and patients with nodal downstaging may still benefit from adjuvant chemotherapy.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Al-Batran S-E, Homann N, Schmalenberg H, Kopp H-G, Haag GM, Luley KB, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): a multicenter, randomized phase 3 trial. J. Clin. Oncol. 2017;35(Suppl.):4004–4004. doi: 10.1200/JCO.2017.35.15_suppl.4004. - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

