Pancreatic cancer cachexia: three dimensions of a complex syndrome
- PMID: 33742145
- PMCID: PMC8110983
- DOI: 10.1038/s41416-021-01301-4
Pancreatic cancer cachexia: three dimensions of a complex syndrome
Abstract
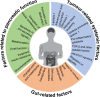
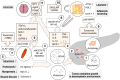
Cancer cachexia is a multifactorial syndrome that is characterised by a loss of skeletal muscle mass, is commonly associated with adipose tissue wasting and malaise, and responds poorly to therapeutic interventions. Although cachexia can affect patients who are severely ill with various malignant or non-malignant conditions, it is particularly common among patients with pancreatic cancer. Pancreatic cancer often leads to the development of cachexia through a combination of distinct factors, which, together, explain its high prevalence and clinical importance in this disease: systemic factors, including metabolic changes and pathogenic signals related to the tumour biology of pancreatic adenocarcinoma; factors resulting from the disruption of the digestive and endocrine functions of the pancreas; and factors related to the close anatomical and functional connection of the pancreas with the gut. In this review, we conceptualise the various insights into the mechanisms underlying pancreatic cancer cachexia according to these three dimensions to expose its particular complexity and the challenges that face clinicians in trying to devise therapeutic interventions.
Conflict of interest statement
M.K.: Alligator Bioscience (Consulting), Roche (Consulting); L.L.: no disclosures; L.E.: no disclosures; J.M.L.: Abbot (Honoraria), Mylan (Honoraria).
Figures

References
-
- Minicozzi P, Cassetti T, Vener C, Sant M. Analysis of incidence, mortality and survival for pancreatic and biliary tract cancers across Europe, with assessment of influence of revised European age standardisation on estimates. Cancer Epidemiol. 2018;55:52–60. doi: 10.1016/j.canep.2018.04.011. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

