Ranibizumab in retinopathy of prematurity - one-year follow-up of ophthalmic outcomes and two-year follow-up of neurodevelopmental outcomes from the CARE-ROP study
- PMID: 33742551
- PMCID: PMC9460412
- DOI: 10.1111/aos.14852
Ranibizumab in retinopathy of prematurity - one-year follow-up of ophthalmic outcomes and two-year follow-up of neurodevelopmental outcomes from the CARE-ROP study
Abstract
Purpose: The primary endpoint results from the comparing alternative ranibizumab dosages for safety and efficacy in retinopathy of prematurity (CARE-ROP) core study identified ranibizumab as an effective treatment to control acute retinopathy of prematurity (ROP). This study reports the 1- and 2-year follow-up data focusing on long-term functional outcomes and safety.
Methods: The CARE-ROP trial compared 0.12 mg versus 0.20 mg ranibizumab in 20 infants with ROP in a multicentric, prospective, randomized, double-blind, controlled study design. Sixteen patients entered the follow-up period. An ophthalmologic assessment at one year postbaseline was acquired from all 16 patients and a neurodevelopmental assessment at two years postbaseline was acquired from 15 patients.
Results: Fifteen of 16 infants were able to fixate and follow moving objects at one year postbaseline treatment. One child progressed to stage 5 ROP bilaterally between the end of the core study and the 1-year follow-up (first seen at PMA 75 weeks). Mean spherical equivalents were -1.9 diopters (D) and -0.75 D in the 0.12 mg and the 0.20 mg treatment arms. Strabismus was present in seven and nystagmus in five out of 16 infants. Mental development scores were within normal limits in six out of ten patients with available data. No statistically significant difference was observed between the two treatment arms.
Conclusion: Neurodevelopmental and functional ocular outcomes 1 and 2 years after treatment with ranibizumab are reassuring regarding long-term safety. Late reactivation of ROP, however, represents a challenge during the follow-up phase and it is of utmost importance that regular follow-ups are maintained.
Keywords: anti-vascular endothelial growth factor; long-term outcomes; ranibizumab; retinopathy of prematurity.
© 2021 The Authors. Acta Ophthalmologica published by John Wiley & Sons Ltd on behalf of Acta Ophthalmologica Scandinavica Foundation.
Figures

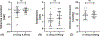

References
-
- Deutsche Ophthalmologische Gesellschaft e. V. (DOG), Retinologische Gesellschaft e. V. (RG) & Berufsverband der Augenärzte Deutschlands e. V. (BVA) (2020, May): Stellungnahme von DOG, RG und BVA zur Anti-VEGF-Therapie der Frühgebore-nenretinopathie; ROP-Pass-Formular zum Download. Available at: https://www.dog.org/wp-content/uploads/2013/03/ROPPass_Version-3_4_FORMU.... (Accessed on 15 Mar 2021).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

