Starvation induces an increase in intracellular calcium and potentiates the progesterone-induced mouse sperm acrosome reaction
- PMID: 33742713
- PMCID: PMC8441833
- DOI: 10.1096/fj.202100122R
Starvation induces an increase in intracellular calcium and potentiates the progesterone-induced mouse sperm acrosome reaction
Abstract
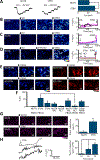
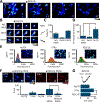
We have recently reported two different methodologies that improve sperm functionality. The first method involved transient exposure to the Ca2+ ionophore A23187 , and the second required sperm incubation in the absence of energy nutrients (starvation). Both methods were associated with an initial loss of motility followed by a rescue step involving ionophore removal or addition of energy metabolites, respectively. In this work, we show that starvation is accompanied by an increase in intracellular Ca2+ ([Ca2+ ]i ). Additionally, the starved cells acquire a significantly enhanced capacity to undergo a progesterone-induced acrosome reaction. Electrophysiological measurements show that CatSper channel remains active in starvation conditions. However, the increase in [Ca2+ ]i was also observed in sperm from CatSper null mice. Upon starvation, addition of energy nutrients reversed the effects on [Ca2+ ]i and decreased the effect of progesterone on the acrosome reaction to control levels. These data indicate that both methods have common molecular features.
Keywords: CatSper; acrosome reaction; intracellular calcium; mice sperm; progesterone; starving.
© 2021 Federation of American Societies for Experimental Biology.
Conflict of interest statement
CONFLICT OF INTERESTS STATEMENT
Dr. Visconti owns equity interest in Sperm Capacitation Technologies Inc. a company with goals in improving assisted reproductive technologies. The others authors have not conflict of interest to declare.
Figures






References
-
- Yanagimachi R The Physiology of Reproduction New York: Knobil; & Neill JD (Eds.);1994. 317 pp.
-
- Gervasi MG, & Visconti PE Chang’s meaning of capacitation: A molecular perspective. Mol Reprod Dev 2016;83:860–874. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

