Safety, tolerability, pharmacokinetics, and pharmacodynamics of a TLR7 agonist prodrug RO6870868 in healthy volunteers
- PMID: 33742764
- PMCID: PMC8301559
- DOI: 10.1111/cts.13016
Safety, tolerability, pharmacokinetics, and pharmacodynamics of a TLR7 agonist prodrug RO6870868 in healthy volunteers
Abstract
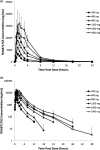
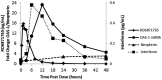
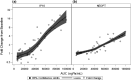
RO6870868 is an oral prodrug of the toll-like receptor 7 (TLR7) specific agonist, RO6871765. TLR7 agonists augment host immune activity and are in development to treat hepatitis B infection. We evaluated the safety, tolerability, pharmacokinetics (PKs), and pharmacodynamics (PDs) of RO6870868 in a first-in-human, phase I, randomized, single ascending oral dose study in 60 healthy volunteers at 6 dose levels (200-2000 mg). Single oral doses were generally well-tolerated with a predictable safety profile associated with dose-dependent increases in systemic interferon. No serious adverse events (AEs) were reported and no subject withdrew from the study due to an AE. No clinically significant changes were observed in vital signs, electrocardiograms, or laboratory parameters. Following oral RO6870868 doses, plasma RO6871765 concentrations increased rapidly, exhibiting mean terminal half-life ranging 2-6 h across all cohorts, with area under the plasma concentration versus time curve extrapolated to infinity (AUC0-∞ ) increasing proportionally with dose. A pattern of dose and time-dependent PD activity was demonstrated consistent with engagement of the TLR7 system. Single RO6870868 doses activated components of the TLR innate immune system in a dose-dependent manner with adequate safety and tolerability. Single-dose data in healthy volunteers are useful to evaluate safety, PK, and PD activity of TLR7 agonists and help to guide dose and regimen selection for further trials in patients with chronic hepatitis B.
© 2021 Deep Dives in Pharma Research, LLC. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of the American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
All authors participated in this study while employed by Hoffmann La Roche and declare no additional competing interests for this work.
Figures


Similar articles
-
A Single and Multiple Ascending Dose Study of Toll-Like Receptor 7 Agonist (RO7020531) in Chinese Healthy Volunteers.Clin Transl Sci. 2020 Sep;13(5):985-993. doi: 10.1111/cts.12791. Epub 2020 May 14. Clin Transl Sci. 2020. PMID: 32268000 Free PMC article. Clinical Trial.
-
Clinical Pharmacokinetics and Safety of ALZ-801, a Novel Prodrug of Tramiprosate in Development for the Treatment of Alzheimer's Disease.Clin Pharmacokinet. 2018 Mar;57(3):315-333. doi: 10.1007/s40262-017-0608-3. Clin Pharmacokinet. 2018. PMID: 29063518 Free PMC article. Clinical Trial.
-
Phase 1 study in healthy participants of the safety, pharmacokinetics, and pharmacodynamics of enpatoran (M5049), a dual antagonist of toll-like receptors 7 and 8.Pharmacol Res Perspect. 2021 Oct;9(5):e00842. doi: 10.1002/prp2.842. Pharmacol Res Perspect. 2021. PMID: 34414672 Free PMC article. Clinical Trial.
-
Safety, Tolerability, and Pharmacokinetics of Single- and Multiple-Ascending Doses of Sunobinop in Healthy Participants.Clin Pharmacol Drug Dev. 2024 Jul;13(7):790-800. doi: 10.1002/cpdd.1394. Epub 2024 Mar 13. Clin Pharmacol Drug Dev. 2024. PMID: 38476082 Clinical Trial.
-
A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects.CNS Drugs. 2018 Nov;32(11):1053-1067. doi: 10.1007/s40263-018-0578-5. CNS Drugs. 2018. PMID: 30374683 Free PMC article. Clinical Trial.
Cited by
-
A TLR7 Agonist Conjugated to a Nanofibrous Peptide Hydrogel as a Potent Vaccine Adjuvant.Adv Healthc Mater. 2025 Jan;14(3):e2402958. doi: 10.1002/adhm.202402958. Epub 2024 Oct 25. Adv Healthc Mater. 2025. PMID: 39460390
-
Using exploratory pharmacokinetic and pharmacodynamic analyses to predict the probability of flu-like symptoms in healthy volunteers and patients with chronic hepatitis B treated with the toll-like receptor 7 agonist ruzotolimod.Clin Transl Sci. 2024 Aug;17(8):e13896. doi: 10.1111/cts.13896. Clin Transl Sci. 2024. PMID: 39119977 Free PMC article. Clinical Trial.
-
Evolution of Toll-like receptor 7/8 agonist therapeutics and their delivery approaches: From antiviral formulations to vaccine adjuvants.Adv Drug Deliv Rev. 2021 Aug;175:113803. doi: 10.1016/j.addr.2021.05.013. Epub 2021 May 29. Adv Drug Deliv Rev. 2021. PMID: 34058283 Free PMC article. Review.
-
Discovery and Characterization of a First-in-Class LIV1-TLR7/8 Immunomodulatory Conjugate with Robust Myeloid Activation and Antitumor Activity.J Med Chem. 2025 Jun 12;68(11):11322-11339. doi: 10.1021/acs.jmedchem.5c00264. Epub 2025 May 24. J Med Chem. 2025. PMID: 40411528 Free PMC article.
-
Involvement of nucleic acid-sensing toll-like receptors in human diseases and their controlling mechanisms.J Biomed Sci. 2025 Jun 10;32(1):56. doi: 10.1186/s12929-025-01151-9. J Biomed Sci. 2025. PMID: 40495154 Free PMC article. Review.
References
-
- World Health Organization . Global hepatitis report 2017. https://www.who.int/hepatitis/publications/global‐hepatitis‐report2017/en/ (2017).
-
- Tang LSY, Covert E, Wilson E, Kottilil S. Chronic hepatitis B infection: a review. JAMA. 2018;319:1802‐1813. - PubMed
-
- [No authors listed]Hepatitis B virus infection. Nat Rev Dis Primers. 2018;4:18036. - PubMed
-
- Ramirez R, Van Buuren N, Suri V. Targeted long read sequencing reveals the comprehensive architecture and expression patterns of integrated HBV DNA in CHB liver biopsies. J Hepatol. 2020;73:S6‐S7.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

