Blood neurofilament light concentration at admittance: a potential prognostic marker in COVID-19
- PMID: 33743046
- PMCID: PMC7980743
- DOI: 10.1007/s00415-021-10517-6
Blood neurofilament light concentration at admittance: a potential prognostic marker in COVID-19
Abstract
Objective: To test the hypotheses that blood biomarkers for nervous system injury, serum concentrations of neurofilament light chain protein (NfL) and glial fibrillary acidic protein (GFAp) can serve as biomarkers for disease severity in COVID-19 patients.
Methods: Forty-seven inpatients with confirmed COVID-19 had blood samples drawn on admission for assessing serum biomarkers of CNS injury by Single molecule array (Simoa), NfL and GFAp. Concentrations of NfL and GFAp were analyzed in relation to symptoms, clinical signs, inflammatory biomarkers and clinical outcomes. We used multivariate linear models to test for differences in biomarker concentrations in the subgroups, accounting for confounding effects.
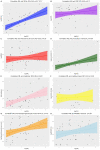
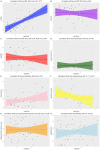
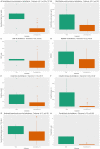
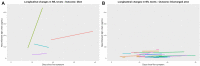
Results: In total, 21% (n = 10) of the patients were admitted to an intensive care unit, and the overall mortality rate was 13% (n = 6). Non-survivors had higher serum concentrations of NfL (p < 0.001) upon admission than patients who were discharged alive both in adjusted analyses (p = 2.6 × 10-7) and unadjusted analyses (p = 0.001). The concentrations of NfL in non-survivors increased over repeated measurements; whereas, the concentrations in survivors were stable. The GFAp concentration was also significantly higher in non-survivors than survivors (p = 0.02).
Conclusion: Increased concentrations of NfL and GFAp in COVID-19 patients on admission may indicate increased mortality risk. Measurement of blood biomarkers for nervous system injury can be useful to detect and monitor CNS injury in COVID-19.
Keywords: COVID-19; Glial fibrillary acidic protein; Mortality; Neurofilament light; SARS-CoV-2.
© 2021. The Author(s).
Conflict of interest statement
The authors report no disclosures relevant to this study. A. H. Aamodt has received travel support, honoraria for advice or lecturing from Bayer, Boehringer Ingelheim, BMS, Allergan, Teva, Sanofi-Genzyme, Novartis, Roche, and Teva and research grant from Medtronic and Boehringer Ingelheim. T. H. Popperud has received honoraria for lecturing from Alexion and unrestricted research support from Octapharma. E. A. Høgestøl has received honoraria for lecturing from Biogen, Merck and Sanofi-Genzyme, and unrestricted research support from Merck and Sanofi-Genzyme. H. F. Harbo has received travel support, honoraria for advice or lecturing from Biogen Idec, Sanofi-Genzyme, Merck, Novartis, Roche, and Teva and an unrestricted research grant from Novartis. Kaj Blennow has served as a consultant, at advisory boards, or at data monitoring committees for Abcam, Axon, Biogen, JOMDD/Shimadzu. Julius Clinical, Lilly, MagQu, Novartis, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. H. Zetterberg has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics and CogRx, has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work).
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

