Delineating the conformational landscape of the adenosine A2A receptor during G protein coupling
- PMID: 33743210
- PMCID: PMC10266235
- DOI: 10.1016/j.cell.2021.02.041
Delineating the conformational landscape of the adenosine A2A receptor during G protein coupling
Abstract
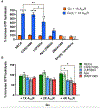
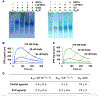
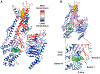
G-protein-coupled receptors (GPCRs) represent a ubiquitous membrane protein family and are important drug targets. Their diverse signaling pathways are driven by complex pharmacology arising from a conformational ensemble rarely captured by structural methods. Here, fluorine nuclear magnetic resonance spectroscopy (19F NMR) is used to delineate key functional states of the adenosine A2A receptor (A2AR) complexed with heterotrimeric G protein (Gαsβ1γ2) in a phospholipid membrane milieu. Analysis of A2AR spectra as a function of ligand, G protein, and nucleotide identifies an ensemble represented by inactive states, a G-protein-bound activation intermediate, and distinct nucleotide-free states associated with either partial- or full-agonist-driven activation. The Gβγ subunit is found to be critical in facilitating ligand-dependent allosteric transmission, as shown by 19F NMR, biochemical, and computational studies. The results provide a mechanistic basis for understanding basal signaling, efficacy, precoupling, and allostery in GPCRs.
Keywords: (19)F NMR; G protein; GPCR; adenosine A(2A) receptor; allostery; partial agonism; precoupling; rigidity transmission allostery.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Comment in
-
Decaf or regular? Energizing the caffeine receptor.Cell. 2021 Apr 1;184(7):1659-1660. doi: 10.1016/j.cell.2021.03.016. Cell. 2021. PMID: 33798438
References
-
- Ballesteros JA, and Weinstein H (1995). Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 25, 366–428.
-
- Ballesteros JA, Jensen AD, Liapakis G, Rasmussen SGF, Shi L, Gether U, and Javitch JA (2001). Activation of the b 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem 276, 29171–29177. - PubMed
-
- Braun S, and Levitzki A (1979). Adenosine receptor permanently coupled to turkey erythrocyte adenylate cyclase. Biochemistry 18, 2134–2138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

