Hitting the diagnostic sweet spot: Point-of-care SARS-CoV-2 salivary antigen testing with an off-the-shelf glucometer
- PMID: 33743492
- PMCID: PMC7908832
- DOI: 10.1016/j.bios.2021.113111
Hitting the diagnostic sweet spot: Point-of-care SARS-CoV-2 salivary antigen testing with an off-the-shelf glucometer
Abstract
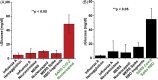
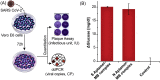
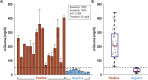
Significant barriers to the diagnosis of latent and acute SARS-CoV-2 infection continue to hamper population-based screening efforts required to contain the COVID-19 pandemic in the absence of widely available antiviral therapeutics or vaccines. We report an aptamer-based SARS-CoV-2 salivary antigen assay employing only low-cost reagents ($3.20/test) and an off-the-shelf glucometer. The test was engineered around a glucometer as it is quantitative, easy to use, and the most prevalent piece of diagnostic equipment globally, making the test highly scalable with an infrastructure that is already in place. Furthermore, many glucometers connect to smartphones, providing an opportunity to integrate with contact tracing apps, medical providers, and electronic health records. In clinical testing, the developed assay detected SARS-CoV-2 infection in patient saliva across a range of viral loads - as benchmarked by RT-qPCR - within 1 h, with 100% sensitivity (positive percent agreement) and distinguished infected specimens from off-target antigens in uninfected controls with 100% specificity (negative percent agreement). We propose that this approach provides an inexpensive, rapid, and accurate diagnostic for distributed screening of SARS-CoV-2 infection at scale.
Keywords: Aptamer; COVID-19; Glucometer; Point-of-care; Population screening; SARS-CoV-2.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- 2019-nCoV Coronavirus Nucleocapsid Elisa Kit KIT40588 | Sino biological. https://www.sinobiological.com/elisa-kits/cov-nucleocapsid-kit40588 [WWW Document], n.d. URL. (accessed 7.12.20)
-
- 2019-nCoV Coronavirus spike Elisa Kit KIT40592 | Sino biological. https://www.sinobiological.com/elisa-kits/cov-spike-kit40592 [WWW Document], n.d. URL. (accessed 7.12.20)
-
- Afzal A. J. Adv. Res. S2090123220301788. 2020
-
- Arevalo-Rodriguez I., Buitrago-Garcia D., Simancas-Racines D., Zambrano-Achig P., del Campo R., Ciapponi A., Sued O., Martinez-Garcia L., Rutjes A., Low N., Bossuyt P.M., Perez-Molina J.A., Zamora J. FALSE-NEGATIVE results OF initial rt-pcr assays for COVID-19: a SYSTEMATIC REVIEW (preprint) Infectious Diseases (except HIV/AIDS) 2020 - PMC - PubMed
-
- Baker B.R., Lai R.Y., Wood M.S., Doctor E.H., Heeger A.J., Plaxco K.W. J. Am. Chem. Soc. 2006;128:3138–3139. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

