Risk factors for repeated dexamethasone intravitreal implant therapy for macular edema due to treatment-naïve branch retinal vein occlusion
- PMID: 33743610
- PMCID: PMC7981849
- DOI: 10.1186/s12886-021-01904-8
Risk factors for repeated dexamethasone intravitreal implant therapy for macular edema due to treatment-naïve branch retinal vein occlusion
Abstract
Background: This study evaluated the effects of dexamethasone intravitreal implant on treatment-naïve branch retinal vein occlusion (BRVO)-induced macular edema (ME), and the risk factors for earlier repeated treatment.
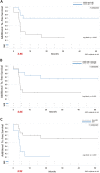
Methods: Patients treated from 2013 to 2016 were enrolled. The patients' demographics, medical history, best-corrected visual acuity (BCVA), and central retinal thickness (CRT) were recorded. Risk factors for repeated treatment were identified using a Cox proportional hazard model and logistic regression.
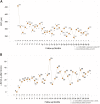
Results: 29 patients (mean age: 58.64 ± 13.3 years) were included; 44.8% received only one injection, while 55.2% received two or more. The mean initial CRT was 457.8 ± 167.1 μm; the peak CRT and final CRT improved significantly to 248.9 ± 57.9 μm and 329.2 ± 115.1 μm, respectively. The peak BCVA improvement and final improvement were 29.5 ± 23.5 approximate ETDRS letters and 19.8 ± 24.4 letters, respectively, with 62.1% of patients improving by more than 15 letters. Older age, higher initial CRT, and diabetes were the risk factors for multiple injections.
Conclusion: Dexamethasone intravitreal implant results in significant peak CRT and BCVA improvements, while older age, higher initial CRT, and diabetes are risk factors for repeated injections. The optimal retreatment schedule for these patients should be further explored.
Keywords: Age; Branch retinal vein occlusion; Central retinal thickness; Dexamethasone intravitreal implant; Diabetes; Macular edema.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Morley MG, Heier JS. Venous obstructive disease of the retina. In: Yanoff M, Duker JS, editors. Ophthalmology. 3. Missouri: Elsevier Mosby; 2009. pp. 597–605.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

