Passive Immunity Trial for Our Nation (PassITON): study protocol for a randomized placebo-control clinical trial evaluating COVID-19 convalescent plasma in hospitalized adults
- PMID: 33743799
- PMCID: PMC7980732
- DOI: 10.1186/s13063-021-05171-2
Passive Immunity Trial for Our Nation (PassITON): study protocol for a randomized placebo-control clinical trial evaluating COVID-19 convalescent plasma in hospitalized adults
Abstract
Background: Convalescent plasma is being used widely as a treatment for coronavirus disease 2019 (COVID-19). However, the clinical efficacy of COVID-19 convalescent plasma is unclear.
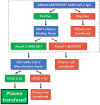
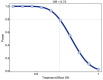
Methods: The Passive Immunity Trial for Our Nation (PassITON) is a multicenter, placebo-controlled, blinded, randomized clinical trial being conducted in the USA to provide high-quality evidence on the efficacy of COVID-19 convalescent plasma as a treatment for adults hospitalized with symptomatic disease. Adults hospitalized with COVID-19 with respiratory symptoms for less than 14 days are eligible. Enrolled patients are randomized in a 1:1 ratio to 1 unit (200-399 mL) of COVID-19 convalescent plasma that has demonstrated neutralizing function using a SARS-CoV-2 chimeric virus neutralization assay. Study treatments are administered in a blinded fashion and patients are followed for 28 days. The primary outcome is clinical status 14 days after study treatment as measured on a 7-category ordinal scale assessing mortality, respiratory support, and return to normal activities of daily living. Key secondary outcomes include mortality and oxygen-free days. The trial is projected to enroll 1000 patients and is designed to detect an odds ratio ≤ 0.73 for the primary outcome.
Discussion: This trial will provide the most robust data available to date on the efficacy of COVID-19 convalescent plasma for the treatment of adults hospitalized with acute moderate to severe COVID-19. These data will be useful to guide the treatment of COVID-19 patients in the current pandemic and for informing decisions about whether developing a standardized infrastructure for collecting and disseminating convalescent plasma to prepare for future viral pandemics is indicated.
Trial registration: ClinicalTrials.gov NCT04362176 . Registered on 24 April 2020.
Keywords: COVID-19; Clinical trials; Neutralizing antibodies; Passive immunity; Randomized controlled trial; SARS-CoV-2: convalescent plasma.
Conflict of interest statement
All authors submitted a competing interest form at the time of manuscript submission. The authors declare that they have no competing interests.
Figures

Update of
-
Passive Immunity Trial for Our Nation (PassITON): study protocol for a randomized placebo-control clinical trial evaluating COVID-19 convalescent plasma in hospitalized adults.Res Sq [Preprint]. 2021 Mar 2:rs.3.rs-227796. doi: 10.21203/rs.3.rs-227796/v1. Res Sq. 2021. Update in: Trials. 2021 Mar 20;22(1):221. doi: 10.1186/s13063-021-05171-2. PMID: 33688640 Free PMC article. Updated. Preprint.
References
-
- WHO Coronavirus Disease (COVID-19) Dashboard. n.d. https://covid19.who.int. Accessed 17 Dec 2020.
-
- Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC, ACTT-1 Study Group Members Remdesivir for the treatment of Covid-19 — final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. - DOI - PMC - PubMed
-
- Pfizer-BioNTech COVID-19 Vaccine. FDA 2020. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise.... Accessed 17 Dec 2020.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

