Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study
- PMID: 33743869
- PMCID: PMC7972311
- DOI: 10.1016/S0140-6736(21)00238-5
Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study
Abstract
Background: Wuhan was the epicentre of the COVID-19 outbreak in China. We aimed to determine the seroprevalence and kinetics of anti-SARS-CoV-2 antibodies at population level in Wuhan to inform the development of vaccination strategies.
Methods: In this longitudinal cross-sectional study, we used a multistage, population-stratified, cluster random sampling method to systematically select 100 communities from the 13 districts of Wuhan. Households were systematically selected from each community and all family members were invited to community health-care centres to participate. Eligible individuals were those who had lived in Wuhan for at least 14 days since Dec 1, 2019. All eligible participants who consented to participate completed a standardised electronic questionnaire of demographic and clinical questions and self-reported any symptoms associated with COVID-19 or previous diagnosis of COVID-19. A venous blood sample was taken for immunological testing on April 14-15, 2020. Blood samples were tested for the presence of pan-immunoglobulins, IgM, IgA, and IgG antibodies against SARS-CoV-2 nucleocapsid protein and neutralising antibodies were assessed. We did two successive follow-ups between June 11 and June 13, and between Oct 9 and Dec 5, 2020, at which blood samples were taken.
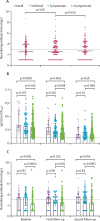
Findings: Of 4600 households randomly selected, 3599 families (78·2%) with 9702 individuals attended the baseline visit. 9542 individuals from 3556 families had sufficient samples for analyses. 532 (5·6%) of 9542 participants were positive for pan-immunoglobulins against SARS-CoV-2, with a baseline adjusted seroprevalence of 6·92% (95% CI 6·41-7·43) in the population. 437 (82·1%) of 532 participants who were positive for pan-immunoglobulins were asymptomatic. 69 (13·0%) of 532 individuals were positive for IgM antibodies, 84 (15·8%) were positive for IgA antibodies, 532 (100%) were positive for IgG antibodies, and 212 (39·8%) were positive for neutralising antibodies at baseline. The proportion of individuals who were positive for pan-immunoglobulins who had neutralising antibodies in April remained stable for the two follow-up visits (162 [44·6%] of 363 in June, 2020, and 187 [41·2%] of 454 in October-December, 2020). On the basis of data from 335 individuals who attended all three follow-up visits and who were positive for pan-immunoglobulins, neutralising antibody levels did not significantly decrease over the study period (median 1/5·6 [IQR 1/2·0 to 1/14·0] at baseline vs 1/5·6 [1/4·0 to 1/11·2] at first follow-up [p=1·0] and 1/6·3 [1/2·0 to 1/12·6] at second follow-up [p=0·29]). However, neutralising antibody titres were lower in asymptomatic individuals than in confirmed cases and symptomatic individuals. Although titres of IgG decreased over time, the proportion of individuals who had IgG antibodies did not decrease substantially (from 30 [100%] of 30 at baseline to 26 [89·7%] of 29 at second follow-up among confirmed cases, 65 [100%] of 65 at baseline to 58 [92·1%] of 63 at second follow-up among symptomatic individuals, and 437 [100%] of 437 at baseline to 329 [90·9%] of 362 at second follow-up among asymptomatic individuals).
Interpretation: 6·92% of a cross-sectional sample of the population of Wuhan developed antibodies against SARS-CoV-2, with 39·8% of this population seroconverting to have neutralising antibodies. Our durability data on humoral responses indicate that mass vaccination is needed to effect herd protection to prevent the resurgence of the epidemic.
Funding: Chinese Academy of Medical Sciences & Peking Union Medical College, National Natural Science Foundation, and Chinese Ministry of Science and Technology.
Translation: For the Chinese translation of the abstract see Supplementary Materials section.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Sustained neutralising antibodies in the Wuhan population suggest durable protection against SARS-CoV-2.Lancet. 2021 Mar 20;397(10279):1037-1039. doi: 10.1016/S0140-6736(21)00434-7. Lancet. 2021. PMID: 33743854 Free PMC article. No abstract available.
References
-
- National Health Commission of the People's Republic of China Daily briefing on novel coronavirus cases in China. Jan 12, 2021. http://www.nhc.gov.cn/xcs/yqtb/202101/b70d608498e549c5973d955e868101e7.s...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

