Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera
- PMID: 33743891
- PMCID: PMC7891044
- DOI: 10.1016/j.cell.2021.02.033
Reduced neutralization of SARS-CoV-2 B.1.1.7 variant by convalescent and vaccine sera
Abstract
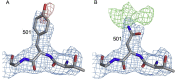
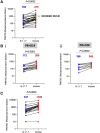
SARS-CoV-2 has caused over 2 million deaths in little over a year. Vaccines are being deployed at scale, aiming to generate responses against the virus spike. The scale of the pandemic and error-prone virus replication is leading to the appearance of mutant viruses and potentially escape from antibody responses. Variant B.1.1.7, now dominant in the UK, with increased transmission, harbors 9 amino acid changes in the spike, including N501Y in the ACE2 interacting surface. We examine the ability of B.1.1.7 to evade antibody responses elicited by natural SARS-CoV-2 infection or vaccination. We map the impact of N501Y by structure/function analysis of a large panel of well-characterized monoclonal antibodies. B.1.1.7 is harder to neutralize than parental virus, compromising neutralization by some members of a major class of public antibodies through light-chain contacts with residue 501. However, widespread escape from monoclonal antibodies or antibody responses generated by natural infection or vaccination was not observed.
Keywords: B.1.1.7; IGHV3-53; Kent; SARS-CoV-2; antibody; escape; neutralization; variant.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests G.R.S. sits on the GSK Vaccines Scientific Advisory Board. Oxford University holds intellectual property related to the Oxford-AstraZeneca vaccine. A.J.P. is Chair of UK Department Health and Social Care’s (DHSC) Joint Committee on Vaccination & Immunisation (JCVI) but does not participate in the JCVI COVID19 committee and is a member of the WHO’s SAGE. S.G. is co-founder of Vaccitech (collaborators in the early development of this vaccine candidate) and named as an inventor on a patent covering use of ChAdOx1-vectored vaccines and a patent application covering this SARS-CoV-2 vaccine. T.L. is named as an inventor on a patent application covering this SARS-CoV-2 vaccine and consultant to Vaccitech. The views expressed in this article do not necessarily represent the views of DHSC, JCVI, or WHO. The University of Oxford has entered into a partnership with AstraZeneca on coronavirus vaccine development. The University of Oxford has protected intellectual property disclosed in this publication.
Figures
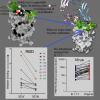
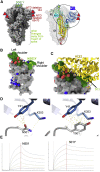
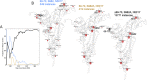
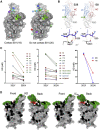



Comment in
-
SARS-CoV-2 variants: a new challenge to convalescent serum and mRNA vaccine neutralization efficiency.Signal Transduct Target Ther. 2021 Apr 10;6(1):151. doi: 10.1038/s41392-021-00592-6. Signal Transduct Target Ther. 2021. PMID: 33839737 Free PMC article. No abstract available.
References
-
- Altmann D.M., Boyton R.J. SARS-CoV-2 T cell immunity: Specificity, function, durability, and role in protection. Sci. Immunol. 2020;5:6160. - PubMed
-
- Aricescu A.R., Lu W., Jones E.Y. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr Sect D Biol Crystallogr. 2006;62:1243–1250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

