Glucocorticoid receptor Thr524 phosphorylation by MINK1 induces interactions with 14-3-3 protein regulators
- PMID: 33744286
- PMCID: PMC8080530
- DOI: 10.1016/j.jbc.2021.100551
Glucocorticoid receptor Thr524 phosphorylation by MINK1 induces interactions with 14-3-3 protein regulators
Abstract
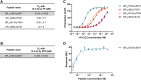
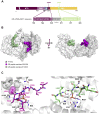
The glucocorticoid receptor (GR) is a ligand-dependent transcription factor that plays a central role in inflammation. The GR activity is also modulated via protein-protein interactions, including binding of 14-3-3 proteins induced by GR phosphorylation. However, the specific phosphorylation sites on the GR that trigger these interactions and their functional consequences are less clear. Hence, we sought to examine this system in more detail. We used phosphorylated GR peptides, biophysical studies, and X-ray crystallography to identify key residues within the ligand-binding domain of the GR, T524 and S617, whose phosphorylation results in binding of the representative 14-3-3 protein 14-3-3ζ. A kinase screen identified misshapen-like kinase 1 (MINK1) as responsible for phosphorylating T524 and Rho-associated protein kinase 1 for phosphorylating S617; cell-based approaches confirmed the importance of both GR phosphosites and MINK1 but not Rho-associated protein kinase 1 alone in inducing GR-14-3-3 binding. Together our results provide molecular-level insight into 14-3-3-mediated regulation of the GR and highlight both MINK1 and the GR-14-3-3 axis as potential targets for future therapeutic intervention.
Keywords: 14-3-3 protein; MINK1 kinase; glucocorticoid receptor; nuclear receptor; phosphorylation; protein–protein interaction.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest L. D. M., K. E., A. G., A. S., L. W., and M. W. D. P. are employed by and/or own shares in AstraZeneca.
Figures


References
-
- Kino T. GR-regulating serine/threonine kinases: New physiologic and pathologic implications. Trends Endocrinol. Metab. 2018;29:260–270. - PubMed
-
- Souffriau J., Eggermont M., Van Ryckeghem S., Van Looveren K., Van Wyngene L., Van Hamme E., Vuylsteke M., Beyaert R., De Bosscher K., Libert C. A screening assay for selective dimerizing glucocorticoid receptor agonists and modulators (SEDIGRAM) that are effective against acute inflammation. Sci. Rep. 2018;8:12894. - PMC - PubMed
-
- Kuna P., Aurivillius M., Jorup C., Prothon S., Taib Z., Edsbäcker S. Efficacy and tolerability of an inhaled selective glucocorticoid receptor modulator – AZD5423 – in chronic obstructive pulmonary disease patients: Phase II study results. Basic Clin. Pharmacol. Toxicol. 2017;121:279–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

