SARS-CoV-2 Fusion Peptide has a Greater Membrane Perturbating Effect than SARS-CoV with Highly Specific Dependence on Ca2
- PMID: 33744314
- PMCID: PMC7969826
- DOI: 10.1016/j.jmb.2021.166946
SARS-CoV-2 Fusion Peptide has a Greater Membrane Perturbating Effect than SARS-CoV with Highly Specific Dependence on Ca2
Abstract
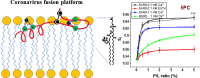
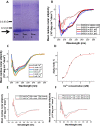
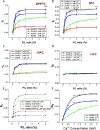
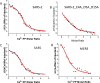
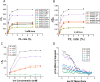
Coronaviruses are a major infectious disease threat, and include the zoonotic-origin human pathogens SARS-CoV-2, SARS-CoV, and MERS-CoV (SARS-2, SARS-1, and MERS). Entry of coronaviruses into host cells is mediated by the spike (S) protein. In our previous ESR studies, the local membrane ordering effect of the fusion peptide (FP) of various viral glycoproteins including the S of SARS-1 and MERS has been consistently observed. We previously determined that the sequence immediately downstream from the S2' cleavage site is the bona fide SARS-1 FP. In this study, we used sequence alignment to identify the SARS-2 FP, and studied its membrane ordering effect. Although there are only three residue differences, SARS-2 FP induces even greater membrane ordering than SARS-1 FP, possibly due to its greater hydrophobicity. This may be a reason that SARS-2 is better able to infect host cells. In addition, the membrane binding enthalpy for SARS-2 is greater. Both the membrane ordering of SARS-2 and SARS-1 FPs are dependent on Ca2+, but that of SARS-2 shows a greater response to the presence of Ca2+. Both FPs bind two Ca2+ ions as does SARS-1 FP, but the two Ca2+ binding sites of SARS-2 exhibit greater cooperativity. This Ca2+ dependence by the SARS-2 FP is very ion-specific. These results show that Ca2+ is an important regulator that interacts with the SARS-2 FP and thus plays a significant role in SARS-2 viral entry. This could lead to therapeutic solutions that either target the FP-calcium interaction or block the Ca2+ channel.
Keywords: COVID-19; ESR; ITC; hydrophobicity; ion specificity.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

