MicroRNA-10 negatively regulates inflammation in diabetic kidney via targeting activation of the NLRP3 inflammasome
- PMID: 33744467
- PMCID: PMC8261077
- DOI: 10.1016/j.ymthe.2021.03.012
MicroRNA-10 negatively regulates inflammation in diabetic kidney via targeting activation of the NLRP3 inflammasome
Abstract
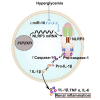
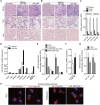
NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome activation has emerged as a central mediator of kidney inflammation in diabetic kidney disease (DKD). However, the mechanism underlying this activation in DKD remains poorly defined. In this study, we found that kidney-enriched microRNA-10a and -10b (miR-10a/b), predominantly expressed in podocytes and tubular epithelial cells, were downregulated in kidney from diabetic mice and patients with DKD. High glucose decreased miR-10a/b expression in vitro in an osmolarity-independent manner. miR-10a/b functioned as negative regulators of the NLRP3 inflammasome through targeting the 3'untranslated region of NLRP3 mRNA, inhibiting assembly of the NLRP3 inflammasome and decreasing caspase-1-dependent release of pro-inflammatory cytokines. Delivery of miR-10a/b into kidney prevented NLRP3 inflammasome activation and renal inflammation, and it reduced albuminuria in streptozotocin (STZ)-treated mice, whereas knocking down miR-10a/b increased NLRP3 inflammasome activation. Restoration of miR-10a/b expression in established DKD ameliorated kidney inflammation and mitigated albuminuria in both db/db and STZ-treated mice. These results suggest a novel intervention strategy for inhibiting kidney inflammation in DKD by targeting the NLRP3 inflammasome.
Keywords: NLRP3 inflammasome; diabetic kidney disease; kidney inflammation; microRNA.
Copyright © 2021 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Declèves A.E., Sharma K. New pharmacological treatments for improving renal outcomes in diabetes. Nat. Rev. Nephrol. 2010;6:371–380. - PubMed
-
- Tang S.C.W., Yiu W.H. Innate immunity in diabetic kidney disease. Nat. Rev. Nephrol. 2020;16:206–222. - PubMed
-
- Wada J., Makino H. Innate immunity in diabetes and diabetic nephropathy. Nat. Rev. Nephrol. 2016;12:13–26. - PubMed
-
- Galkina E., Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J. Am. Soc. Nephrol. 2006;17:368–377. - PubMed
-
- Wada J., Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin. Sci. (Lond.) 2013;124:139–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

