Targeting of miR-33 ameliorates phenotypes linked to age-related macular degeneration
- PMID: 33744470
- PMCID: PMC8261163
- DOI: 10.1016/j.ymthe.2021.03.014
Targeting of miR-33 ameliorates phenotypes linked to age-related macular degeneration
Abstract
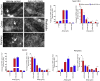
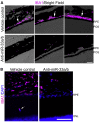
Abnormal cholesterol/lipid homeostasis is linked to neurodegenerative conditions such as age-related macular degeneration (AMD), which is a leading cause of blindness in the elderly. The most prevalent form, termed "dry" AMD, is characterized by pathological cholesterol accumulation beneath the retinal pigment epithelial (RPE) cell layer and inflammation-linked degeneration in the retina. We show here that the cholesterol-regulating microRNA miR-33 was elevated in the RPE of aging mice. Expression of the miR-33 target ATP-binding cassette transporter (ABCA1), a cholesterol efflux pump genetically linked to AMD, declined reciprocally in the RPE with age. In accord, miR-33 modulated ABCA1 expression and cholesterol efflux in human RPE cells. Subcutaneous delivery of miR-33 antisense oligonucleotides (ASO) to aging mice and non-human primates fed a Western-type high fat/cholesterol diet resulted in increased ABCA1 expression, decreased cholesterol accumulation, and reduced immune cell infiltration in the RPE cell layer, accompanied by decreased pathological changes to RPE morphology. These findings suggest that miR-33 targeting may decrease cholesterol deposition and ameliorate AMD initiation and progression.
Keywords: ABCA1; age-related macular degeneration; cholesterol; geographic atrophy; inflammation; microRNA; retinal pigment epithelial cells.
Copyright © 2021 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

