Clinical evaluation of the antibody response in patients with COVID-19 using automated high-throughput immunoassays
- PMID: 33744623
- PMCID: PMC7954771
- DOI: 10.1016/j.diagmicrobio.2021.115370
Clinical evaluation of the antibody response in patients with COVID-19 using automated high-throughput immunoassays
Abstract
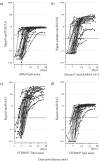
Several automated high-throughput immunoassays for detecting anti-SARS-CoV-2 antibodies by a semi-quantitative approach have been commercialized. In this study, we describe the timeline of the antibody response in patients with RT-PCR-confirmed COVID-19. A total of 292 sequential serum samples from 33 Japanese patients were retrospectively analyzed using four test kits for SARS-CoV-2: the Abbott SARS-CoV-2 IgG assay (Abbott), Elecsys® Anti-SARS-CoV-2 assay (Roche Diagnostic), and VITROS® Anti-SARS-CoV-2 Total and IgG assays (Ortho Clinical Diagnostics). All automated immunoassays could equivalently identify positive sera collected within 2 weeks after symptom onset (99.3%-100%). In addition, the S protein-based automated immunoassay, the VITROS® Anti-SARS-CoV-2 Total assay, may play a complementary role in evaluating passive antibody therapies or vaccines against SARS-CoV-2, although further research is required.
Keywords: Antibodies; CLIA; COVID-19; ECLIA; Immunoassay; SARS-cov-2.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no competing interests.
Figures
Similar articles
-
Head-to-head comparison of two rapid high-throughput automated electrochemiluminescence immunoassays targeting total antibodies to the SARS-CoV-2 nucleoprotein and spike protein receptor binding domain.J Clin Virol. 2021 Apr;137:104784. doi: 10.1016/j.jcv.2021.104784. Epub 2021 Mar 5. J Clin Virol. 2021. PMID: 33711693 Free PMC article.
-
Recent Advances in the Evaluation of Serological Assays for the Diagnosis of SARS-CoV-2 Infection and COVID-19.Front Public Health. 2021 Feb 18;8:620222. doi: 10.3389/fpubh.2020.620222. eCollection 2020. Front Public Health. 2021. PMID: 33681115 Free PMC article.
-
Comparison of four high-throughput, automated immunoassays for the detection of SARS-CoV-2 antibodies.Ann Clin Biochem. 2021 Sep;58(5):487-495. doi: 10.1177/00045632211015711. Epub 2021 Jun 2. Ann Clin Biochem. 2021. PMID: 33892600
-
How to interpret and use COVID-19 serology and immunology tests.Clin Microbiol Infect. 2021 Jul;27(7):981-986. doi: 10.1016/j.cmi.2021.05.001. Epub 2021 May 8. Clin Microbiol Infect. 2021. PMID: 33975005 Free PMC article. Review.
-
Diagnostic Accuracy Estimates for COVID-19 Real-Time Polymerase Chain Reaction and Lateral Flow Immunoassay Tests With Bayesian Latent-Class Models.Am J Epidemiol. 2021 Aug 1;190(8):1689-1695. doi: 10.1093/aje/kwab093. Am J Epidemiol. 2021. PMID: 33823529 Free PMC article. Review.
Cited by
-
Effectiveness of COVID-19 vaccination in healthcare workers in Shiga Prefecture, Japan.Sci Rep. 2022 Oct 21;12(1):17621. doi: 10.1038/s41598-022-22682-3. Sci Rep. 2022. PMID: 36271136 Free PMC article.
-
Recent advances in point of care testing for COVID-19 detection.Biomed Pharmacother. 2022 Sep;153:113538. doi: 10.1016/j.biopha.2022.113538. Epub 2022 Aug 12. Biomed Pharmacother. 2022. PMID: 36076617 Free PMC article. Review.
-
Diagnostic Efficacy of 11 SARS-CoV-2 Serological Assays for COVID-19: A Meta-Analysis and Adjusted Indirect Comparison of Diagnostic Test Accuracy.Immun Inflamm Dis. 2024 Dec;12(12):e70114. doi: 10.1002/iid3.70114. Immun Inflamm Dis. 2024. PMID: 39698931 Free PMC article.
-
In-depth immunochemical characterization of the serum antibody response using a dual-titration microspot assay.Front Immunol. 2025 Feb 25;16:1494624. doi: 10.3389/fimmu.2025.1494624. eCollection 2025. Front Immunol. 2025. PMID: 40070838 Free PMC article.
-
Serology suggests adequate safety measures to protect healthcare workers from COVID-19 in Shiga Prefecture, Japan.PLoS One. 2022 Jun 24;17(6):e0270334. doi: 10.1371/journal.pone.0270334. eCollection 2022. PLoS One. 2022. PMID: 35749426 Free PMC article.
References
-
- China National Health Commission. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition). http://kjfy.meetingchina.org/msite/news/show/cn/3337.html. Accessed July 12, 2020.
-
- Coronavirus disease (COVID-19) pandemic, WHO. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed August 9, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

