The prognostic value of circulating tumor DNA in patients with melanoma: A systematic review and meta-analysis
- PMID: 33744725
- PMCID: PMC7985561
- DOI: 10.1016/j.tranon.2021.101072
The prognostic value of circulating tumor DNA in patients with melanoma: A systematic review and meta-analysis
Abstract
Background: Circulating tumor DNA (ctDNA) has been investigated as a potential prognostic biomarker to evaluate the therapeutic efficacy and disease progression in melanoma patients, yet results remain inconclusive. The purpose of this study was to illustrate the prognostic value of ctDNA in melanoma.
Objectives: To describe the clinical prognostic value of ctDNA for melanoma patients.
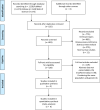
Methods: Searched for eligible articles from Pubmed, Web of Science and Embase. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated to evaluate the association between ctDNA at baseline or during treatment and overall survival (OS) and progression-free survival (PFS).
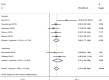
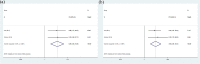
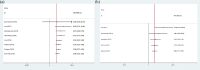
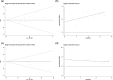
Results: A total of 9 articles were obtained, involving 617 melanoma patients. The pooled HRs revealed that compared with baseline undetectable ctDNA patients, detectable ctDNA was highly correlated with poor OS (HR 2.91, 95% CI: 2.22-3.82; p < 0.001) and PFS (HR 2.75, 95% CI: 1.98-3.83; p < 0.001). A meta-analysis of these adjusted HRs was performed and confirmed that ctDNA collected at baseline was associated with poorer OS/PFS (OS: HR 3.00, 95% CI 2.19-4.11, p < 0.001/PFS: HR 2.68, 95% CI 1.77-4.06, p < 0.001). During treatment, a significant association was shown between ctDNA and poorer OS/PFS (OS: HR 6.26, 95% CI 2.48-15.80, p < 0.001; PFS: HR 4.93, 95% CI 2.36-10.33, p < 0.001).
Conclusion: Investigation and application of ctDNA will improve "liquid biopsy" and play a role in early prediction, monitoring disease progression and precise adjusting treatment strategies in melanoma patients.
Keywords: Circulating tumor DNA; Melanoma; Meta-analysis; Prognostic value.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- DePeralta D.K., Boland G.M. Melanoma: advances in targeted therapy and molecular markers. Ann. Surg. Oncol. 2015;22:3451–3458. - PubMed
-
- Oliveira K.C.S., Ramos I.B., Silva J.M.C., Barra W.F., Riggins G.J., Palande V. Current perspectives on circulating tumor DNA, precision medicine, and personalized clinical management of cancer. Molecul. Cancer Res.: MCR. 2020;18:517–528. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

