A randomized phase IIa study of quantified bone scan response in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with radium-223 dichloride alone or in combination with abiraterone acetate/prednisone or enzalutamide
- PMID: 33744812
- PMCID: PMC7985394
- DOI: 10.1016/j.esmoop.2021.100082
A randomized phase IIa study of quantified bone scan response in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with radium-223 dichloride alone or in combination with abiraterone acetate/prednisone or enzalutamide
Abstract
Background: In metastatic castration-resistant prostate cancer (mCRPC), assessing treatment response and bone lesions with technetium-99m is limited by image resolution and subjectivity. We evaluated bone scan lesion area (BSLA), a quantitative imaging assessment of response in patients with mCRPC receiving radium-223 alone or in combination with androgen receptor pathway inhibitors (abiraterone/prednisone or enzalutamide).
Patients and methods: This randomized, non-comparative phase IIa three-arm trial (NCT02034552) evaluated technetium-99m-based BSLA response rate (RR), safety, radiologic progression-free survival (rPFS), and time to first symptomatic skeletal event (SSE) in men with mCRPC and bone metastases receiving radium-223 with/without abiraterone/prednisone or enzalutamide. The primary endpoint was week 24 BSLA RR.
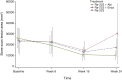
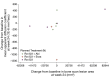
Results: Overall, 63 patients received treatment (abiraterone/prednisone combination, n = 22; enzalutamide combination, n = 22; radium-223 monotherapy, n = 19). Median treatment duration (first to last dose of any study treatment) was 12 months (abiraterone/prednisone combination), 10 months (enzalutamide combination), and 3 months (radium-223 monotherapy). Week 24 BSLA RR was 58% [80% confidence interval (CI) 41% to 74%; one-sided P < 0.0001; 11/19 patients] with abiraterone/prednisone combination, 50% (32% to 68%; one-sided P < 0.0001; 8/16 patients) with enzalutamide combination, and 22% (10% to 40%; one-sided P = 0.0109; 4/18 patients) with radium-223 monotherapy. Median rPFS was not evaluable for combination arms and 4 months (80% CI 4 to 12) for monotherapy. SSEs were reported in 32% of patients; median time to first SSE was not estimable. Fatigue and back pain were the most commonly reported treatment-emergent adverse events (TEAEs); more patients receiving combination therapy than monotherapy had TEAEs. Fractures were reported in 18% receiving abiraterone/prednisone, 32% receiving enzalutamide, and 11% receiving radium-223 monotherapy. Fracture rates were lower in patients taking bone health agents versus not taking bone health agents at baseline.
Conclusions: Technetium-99m imaging BSLA may offer objective, quantifiable assessment of isotope uptake changes, and potentially treatment response, in patients with mCRPC and bone metastases treated with radium-223 alone or in combination with abiraterone/prednisone or enzalutamide. In this largely treatment-naive population, BSLA RR was numerically lower with radium-223 monotherapy versus combination therapy, indicating a limited role as first-line treatment. Use of radium-223 should follow evidence-based treatment guidelines and the licensed indication.
Keywords: abiraterone; bone scan lesion area; enzalutamide; metastatic castration-resistant prostate cancer; radium-223; technetium-99m.
Copyright © 2021. Published by Elsevier Ltd.
Conflict of interest statement
Disclosure DPP has received honoraria from Advanced Accelerator Applications, Amgen, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Clovis Oncology, Eli Lilly, Exelixis, Incyte, Janssen, Pfizer, Pharmacyclics, Roche, Seattle Genetics, and UroGen; and has received research grants/funding from Advanced Accelerator Applications, Astellas, AstraZeneca, Bayer, Bristol-Myers Squibb, Clovis Oncology, Eli Lilly, Endocyte, Genentech, Innocrin, MedImmune, Merck, Novartis, Pfizer, Progenics, Roche, Sanofi Aventis, and Seattle Genetics. UNV has received honoraria, has acted as an advisor/consultant, and has received research grants/funding from Bayer, Sanofi Inc., Exelixis, Bristol-Myers Squibb, Pfizer, and EMD Serono Inc. CSH has acted as an advisor/consultant for Aptevo, Asana, Astellas, AstraZeneca, Bayer, Blue Earth Diagnostics, Churchill Pharma, Clovis Oncology, Dendreon, Endocyte, Ferring, Hinova Pharma, Janssen, Myriad Genetics, Orion Corporation, and Pfizer; has received research grants/funding from Aptevo, Bayer, Aragon Pharma, Astellas, AstraZeneca, Dendreon, Genentech, Hoffman-La Roche, Medivation, Sanofi, and Pfizer; and has received travel, accommodation, and expenses from Bayer, Blue Earth Diagnostics, Clovis Oncology, Ferring, Genentech, Hinova, Janssen, Myriad Genetics, Orion Corporation, and Pfizer. CA has acted as a speaker bureau/expert testimony for Sanofi. NAD has acted as a speaker bureau/expert testimony for Astellas/Pfizer and Janssen. BAM has received honoraria, travel, and accommodation expenses, and acted as an advisor/consultant for Astellas, Amgen, Bayer, Janssen, and Pfizer; and has received grants/funding from Astellas, Bayer, Janssen, and Pfizer. DIQ has received research grants/funding from Seattle Genetics, MSD, and Novartis; has acted as an advisor/consultant for Astellas, AstraZeneca, Bayer, BMS, Dendreon, Exelixis, Roche, Janssen, MSD, Novartis, Pfizer, and Sanofi; and has received travel, accommodation, and expenses from Pfizer, MSD, AstraZeneca, BMS, and Roche. OS reports grand and/or fees from Advanced Accelerator Applications, Astellas, AstraZeneca, Bayer, Bellicum, Blue Earth Diagnostics, Inc., Bristol-Myers Squibb, Celgene, Constellation, Dendreon, EMD Serono, Innocrin, Invitae, Johnson & Johnson, Merck, Myovant, Pfizer, Sanofi, and SOTIO. VJW, JS, and LT are employees of Bayer HealthCare Pharmaceuticals. All other authors have declared no conflicts of interest.
Figures


References
-
- Lorente D., Fizazi K., Sweeney C., de Bono J.S. Optimal treatment sequence for metastatic castration-resistant prostate cancer. Eur Urol Focus. 2016;2:488–498. - PubMed
-
- Bruland O.S., Nilsson S., Fisher D.R., Larsen R.H. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res. 2006;12:6250s–6257s. - PubMed
-
- Scher H.I., Fizazi K., Saad F. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

