BMP5 silencing inhibits chondrocyte senescence and apoptosis as well as osteoarthritis progression in mice
- PMID: 33744859
- PMCID: PMC8064147
- DOI: 10.18632/aging.202708
BMP5 silencing inhibits chondrocyte senescence and apoptosis as well as osteoarthritis progression in mice
Abstract
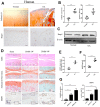
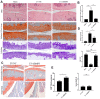
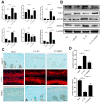
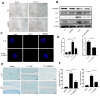
In this study, we using the in vivo destabilization of the medial meniscus (DMM) mouse model to investigate the role of bone morphogenetic protein 5 (BMP5) in osteoarthritis (OA) progression mediated via chondrocyte senescence and apoptosis. BMP5 expression was significantly higher in knee articular cartilage tissues of OA patients and DMM model mice than the corresponding controls. The Osteoarthritis Research Society International scores based on histological staining of knee articular cartilage sections were lower in DMM mice where BMP5 was knocked down in chondrocytes than the corresponding controls 4 weeks after DMM surgery. DMM mice with BMP5-deficient chondrocytes showed reduced levels of matrix-degrading enzymes such as MMP13 and ADAMTS5 as well as reduced cartilage destruction. BMP5 knockdown also decreased chondrocyte apoptosis and senescence by suppressing the activation of p38 and ERK MAP kinases. These findings demonstrate that BMP5 silencing inhibits chondrocyte senescence and apoptosis as well as OA progression by downregulating activity in the p38/ERK signaling pathway.
Keywords: BMP5; chondrocyte; osteoarthritis; p38/ERK; senescence.
Conflict of interest statement
Figures

References
-
- Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, Shah M, Thompson EW. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009; 60:3723–33. 10.1002/art.25002 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

