Revisiting Co-trimoxazole Prophylaxis for African Adults in the Era of Antiretroviral Therapy: A Randomized Controlled Clinical Trial
- PMID: 33744963
- PMCID: PMC8442771
- DOI: 10.1093/cid/ciab252
Revisiting Co-trimoxazole Prophylaxis for African Adults in the Era of Antiretroviral Therapy: A Randomized Controlled Clinical Trial
Abstract
Background: Daily co-trimoxazole is recommended for African adults living with human immunodeficiency virus (HIV) irrespective of antiretroviral treatment, immune status, or disease stage. Benefits of continued prophylaxis and whether co-trimoxazole can be stopped following immune reconstitution are unknown.
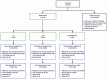
Methods: We conducted a randomized controlled trial at 2 sites in Malawi that enrolled adults with HIV with undetectable viral load and CD4 count of >250/mm3 and randomized them to continue daily co-trimoxazole, discontinue daily co-trimoxazole and begin weekly chloroquine, or discontinue daily co-trimoxazole. The primary endpoint was the preventive effect of co-trimoxazole prophylaxis against death or World Health Organization (WHO) HIV/AIDS stage 3-4 events, using Cox proportional hazards modeling, in an intention-to-treat population.
Results: 1499 adults were enrolled. The preventive effect of co-trimoxazole on the primary endpoint was 22% (95% CI: -14%-47%; P = .20) versus no prophylaxis and 25% (-10%-48%; P = .14) versus chloroquine. When WHO HIV/AIDS stage 2 events were added to the primary endpoint, preventive effect increased to 31% (3-51%; P = .032) and 32% (4-51%; P = .026), respectively. Co-trimoxazole and chloroquine prophylaxis effectively prevented clinical malaria episodes (3.8 and 3.0, respectively, vs 28/100 person-years; P < .001).
Conclusions: Malawian adults with HIV who immune reconstituted on ART and continued co-trimoxazole prophylaxis experienced fewer deaths and WHO HIV/AIDS stage 3-4 events compared with prophylaxis discontinuation, although statistical significance was not achieved. Co-trimoxazole prevented a composite of death plus WHO HIV/AIDS stage 2-4 events. Given poor healthcare access and lack of routine viral load monitoring, co-trimoxazole prophylaxis should continue in adults on ART after immune reconstitution in sub-Saharan Africa. Clinical Trials Registration. NCT01650558.
Keywords: Africa; HIV infection; chloroquine; malaria; trimethoprim-sulfamethoxazole.
© The Author(s) 2021. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

Comment in
-
Using a Composite Outcome in Estimating the Effect of Co-trimoxazole Prophylaxis on Prognosis in African Adults Receiving Antiretroviral Therapy.Clin Infect Dis. 2021 Oct 20;73(8):1550-1551. doi: 10.1093/cid/ciab362. Clin Infect Dis. 2021. PMID: 33900382 No abstract available.
-
Reply to Ramirez and Diaz-Quijano.Clin Infect Dis. 2021 Oct 20;73(8):1551-1552. doi: 10.1093/cid/ciab364. Clin Infect Dis. 2021. PMID: 33900389 Free PMC article. No abstract available.
References
-
- Anglaret X, Chêne G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d’Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet 1999; 353:1463–8. - PubMed
-
- Wiktor SZ, Sassan-Morokro M, Grant AD, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Côte d’Ivoire: a randomised controlled trial. Lancet 1999; 353:1469–75. - PubMed
-
- Mermin J, Lule J, Ekwaru JP, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet 2004; 364:1428–34. - PubMed
-
- Chintu C, Bhat GJ, Walker AS, et al. ; CHAP Trial Team . Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet 2004; 364:1865–71. - PubMed
-
- Mussini C, Pezzotti P, Antinori A, et al. ; Changes in Opportunistic Prophylaxis (CIOP) Study Group . Discontinuation of secondary prophylaxis for Pneumocystis carinii pneumonia in human immunodeficiency virus-infected patients: a randomized trial by the CIOP Study Group. Clin Infect Dis 2003; 36:645–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

