A Positive Feedback Loop Mediated by CsERF31 Initiates Female Cucumber Flower Development: ETHYLENE RESPONSE FACTOR31 mediates a positive feedback loop that initiates female cucumber flower development
- PMID: 33744968
- PMCID: PMC8195516
- DOI: 10.1093/plphys/kiab141
A Positive Feedback Loop Mediated by CsERF31 Initiates Female Cucumber Flower Development: ETHYLENE RESPONSE FACTOR31 mediates a positive feedback loop that initiates female cucumber flower development
Abstract
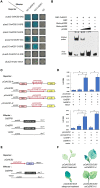
Sex determination is a crucially important developmental event that is pervasive throughout nature and enhances the adaptation of species. Among plants, cucumber (Cucumis sativus L.) can generate both unisexual and bisexual flowers, and the sex type is mainly controlled by several 1-aminocyclopropane-1-carboxylic acid (ACC) synthases. However, the regulatory mechanism of these synthases remains elusive. Here, we used gene expression analysis, protein-DNA interaction assays and transgenic plants to study the function of a gynoecium-specific gene, ETHYLENE RESPONSE FACTOR31 (CsERF31), in female flower differentiation. We found that in a predetermined female flower, ethylene signalling activates CsERF31 by CsEIN3, and then CsERF31 stimulates CsACS2, which triggers a positive feedback loop to ensure female rather than bisexual flower development. A similar interplay is functionally conserved in melon (Cucumis melo L.). Knockdown of CsERF31 by RNAi causes defective bisexual flowers to replace female flowers. Ectopic expression of CsERF31 suppresses stamen development and promotes pistil development in male flowers, demonstrating that CsERF31 functions as a sex switch. Taken together, our data confirm that CsERF31 represents the molecular link between female-male determination and female-bisexual determination, and provide mechanistic insight into how ethylene promotes female flowers, rather than bisexual flowers, in cucumber sex determination.
Keywords: ERF; cucumber; ethylene signalling; positive-feedback; sex determination; unisexual flower.
© The Author(s) 2021. Published by Oxford University Press on behalf of American Society of Plant Biologists. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
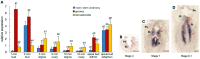




References
-
- Bai SL, Peng YB, Cui JX, Gu HT, Xu LY, Li YQ, Xu ZH, Bai SN (2004) Developmental analyses reveal early arrests of the spore-bearing parts of reproductive organs in unisexual flowers of cucumber (Cucumis sativus L). Planta 220: 230–240 - PubMed
-
- Bai SN, Xu ZH (2012) Bird–nest puzzle: can the study of unisexual flowers such as cucumber solve the problem of plant sex determination? Protoplasma 249: 119–123 - PubMed
-
- Boualem A, Fergany M, Fernandez R, Troadec C, Martin A, Morin H, Sari MA, Collin F, Flowers JM, Pitrat M, et al. (2008) A conserved mutation in an ethylene biosynthesis enzyme leads to andromonoecy in melons. Science 321: 836–838 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

