A Comprehensive Phylogenetic Analysis of the Serpin Superfamily
- PMID: 33744972
- PMCID: PMC8233489
- DOI: 10.1093/molbev/msab081
A Comprehensive Phylogenetic Analysis of the Serpin Superfamily
Abstract
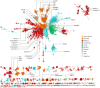
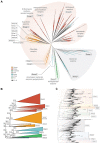
Serine protease inhibitors (serpins) are found in all kingdoms of life and play essential roles in multiple physiological processes. Owing to the diversity of the superfamily, phylogenetic analysis is challenging and prokaryotic serpins have been speculated to have been acquired from Metazoa through horizontal gene transfer due to their unexpectedly high homology. Here, we have leveraged a structural alignment of diverse serpins to generate a comprehensive 6,000-sequence phylogeny that encompasses serpins from all kingdoms of life. We show that in addition to a central "hub" of highly conserved serpins, there has been extensive diversification of the superfamily into many novel functional clades. Our analysis indicates that the hub proteins are ancient and are similar because of convergent evolution, rather than the alternative hypothesis of horizontal gene transfer. This work clarifies longstanding questions in the evolution of serpins and provides new directions for research in the field of serpin biology.
Keywords: evolution; phylogenetics; serpin.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
Figures



References
-
- Ahmed FH, Hafna Ahmed F, Carr PD, Lee BM, Afriat-Jurnou L, Elaaf Mohamed A, Hong N-S, Flanagan J, Taylor MC, Greening C.. 2015. Sequence–structure–function classification of a catalytically diverse oxidoreductase superfamily in mycobacteria. J Mol Biol. 427(22):3554–3571. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

