Angiodiversity and organotypic functions of sinusoidal endothelial cells
- PMID: 33745018
- PMCID: PMC7982081
- DOI: 10.1007/s10456-021-09780-y
Angiodiversity and organotypic functions of sinusoidal endothelial cells
Abstract
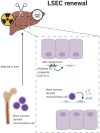
'Angiodiversity' refers to the structural and functional heterogeneity of endothelial cells (EC) along the segments of the vascular tree and especially within the microvascular beds of different organs. Organotypically differentiated EC ranging from continuous, barrier-forming endothelium to discontinuous, fenestrated endothelium perform organ-specific functions such as the maintenance of the tightly sealed blood-brain barrier or the clearance of macromolecular waste products from the peripheral blood by liver EC-expressed scavenger receptors. The microvascular bed of the liver, composed of discontinuous, fenestrated liver sinusoidal endothelial cells (LSEC), is a prime example of organ-specific angiodiversity. Anatomy and development of LSEC have been extensively studied by electron microscopy as well as linage-tracing experiments. Recent advances in cell isolation and bulk transcriptomics or single-cell RNA sequencing techniques allowed the identification of distinct LSEC molecular programs and have led to the identification of LSEC subpopulations. LSEC execute homeostatic functions such as fine tuning the vascular tone, clearing noxious substances from the circulation, and modulating immunoregulatory mechanisms. In recent years, the identification and functional analysis of LSEC-derived angiocrine signals, which control liver homeostasis and disease pathogenesis in an instructive manner, marks a major change of paradigm in the understanding of liver function in health and disease. This review summarizes recent advances in the understanding of liver vascular angiodiversity and the functional consequences resulting thereof.
Keywords: Angiocrine signaling; Angiodiversity; Endothelial cell heterogeneity; Liver vasculature; Sinusoids.
Figures






References
-
- Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100(2):174–190. doi: 10.1161/01.RES.0000255690.03436.ae. - DOI - PubMed
-
- Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O, Rafii S. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell. 2013;26(2):204–219. doi: 10.1016/j.devcel.2013.06.017. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

