Implications of the RhoA/Rho associated kinase pathway and leptin in primary uterine inertia in the dog
- PMID: 33746146
- PMCID: PMC8238673
- DOI: 10.1262/jrd.2020-141
Implications of the RhoA/Rho associated kinase pathway and leptin in primary uterine inertia in the dog
Abstract
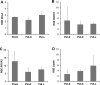
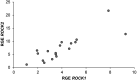
The underlying functional and molecular changes in canine primary uterine inertia (PUI) are still not clarified. Leptin (Lep) and obesity negatively affect uterine contractility in women, partly mediated by the RhoA/Rho associated kinase pathway, affecting myometrial calcium sensitization. We hypothesized that increased uterine Lep/Lep receptor (LepR) or decreased RhoA/Rho associated kinase expression contributes to PUI in dogs, independent of obesity. Dogs presented for dystocia were grouped into PUI (n = 11) or obstructive dystocia (OD, still showing strong labor contractions; n = 7). Interplacental full-thickness uterine biopsies were collected during Cesarean section for relative gene expression (RGE) of RhoA, its effector kinases (ROCK1, ROCK2), Lep and LepR by qPCR. Protein and/or mRNA expression and localization was evaluated by immunohistochemistry and in situ hybridization. RGE was compared between groups by one-way ANOVA using body weight as covariate with statistical significance at P < 0.05. Uterine ROCK1 and ROCK2 gene expression was significantly higher in PUI than OD, while RhoA and Lep did not differ. LepR RGE was below the detection limit in five PUI and all OD dogs. Litter size had no influence. Lep, LepR, RhoA, ROCK1, ROCK2 protein and/or mRNA were localized in the myometrium and endometrium. Uterine protein expression appeared similar between groups. LepR mRNA signals appeared stronger in PUI than OD. In conclusion, lasting, strong labor contractions in OD likely resulted in downregulation of uterine ROCK1 and ROCK2, contrasting the higher expression in PUI dogs with insufficient contractions. The Lep-LepR system may affect uterine contractility in non-obese PUI dogs in a paracrine-autocrine manner.
Keywords: Canine; Contractility; Dystocia; Parturition; Uterus.
Figures




Similar articles
-
Uterine expression of smooth muscle alpha- and gamma-actin and smooth muscle myosin in bitches diagnosed with uterine inertia and obstructive dystocia.Theriogenology. 2020 Oct 15;156:162-170. doi: 10.1016/j.theriogenology.2020.06.033. Epub 2020 Jun 30. Theriogenology. 2020. PMID: 32750597
-
Investigations on the potential role of prostaglandin E2 in canine uterine inertia.Theriogenology. 2021 Nov;175:134-147. doi: 10.1016/j.theriogenology.2021.09.003. Epub 2021 Sep 10. Theriogenology. 2021. PMID: 34544012
-
Leptin in the canine uterus and placenta: possible implications in pregnancy.Reprod Biol Endocrinol. 2015 Mar 8;13:13. doi: 10.1186/s12958-015-0003-6. Reprod Biol Endocrinol. 2015. PMID: 25871422 Free PMC article.
-
The Myometrium in Pregnant Women with Obesity.Curr Vasc Pharmacol. 2021;19(2):193-200. doi: 10.2174/1570161118666200525133530. Curr Vasc Pharmacol. 2021. PMID: 32484103 Review.
-
RhoA/Rho-Kinase in the Cardiovascular System.Circ Res. 2016 Jan 22;118(2):352-66. doi: 10.1161/CIRCRESAHA.115.306532. Circ Res. 2016. PMID: 26838319 Review.
Cited by
-
The In Vitro Contractile Response of Canine Pregnant Myometrium to Oxytocin and Denaverine Hydrochloride.Biology (Basel). 2023 Jun 15;12(6):860. doi: 10.3390/biology12060860. Biology (Basel). 2023. PMID: 37372145 Free PMC article.
-
Insights into the role of PGF2α in canine periparturient myometrium.Front Physiol. 2024 May 28;15:1392080. doi: 10.3389/fphys.2024.1392080. eCollection 2024. Front Physiol. 2024. PMID: 38863475 Free PMC article.
-
Involvement of Oxytocin and Progesterone Receptor Expression in the Etiology of Canine Uterine Inertia.Int J Mol Sci. 2022 Nov 6;23(21):13601. doi: 10.3390/ijms232113601. Int J Mol Sci. 2022. PMID: 36362391 Free PMC article.
References
-
- Johnson C. False pregnancy, disorders of pregnancy and parturition, and mismating. In: Nelson RW, Couto CG (eds.), Small Animal Internal Medicine. St. Louis, MO: Mosby Elsevier; 2009: 931-935.
-
- Bennett D. Canine dystocia—a review of the literature. J Small Anim Pract 1974; 15: 101–117. - PubMed
-
- Darvelid AW, Linde-Forsberg C. Dystocia in the bitch: A retrospective study of 182 cases. J Small Anim Pract 1994; 35: 402–407.
-
- Davidson AP. Primary uterine inertia in four labrador bitches. J Am Anim Hosp Assoc 2011; 47: 83–88. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

