SARS COV-2 virus-laden droplets coughed from deep lungs: Numerical quantification in a single-path whole respiratory tract geometry
- PMID: 33746489
- PMCID: PMC7976054
- DOI: 10.1063/5.0040914
SARS COV-2 virus-laden droplets coughed from deep lungs: Numerical quantification in a single-path whole respiratory tract geometry
Abstract
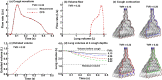
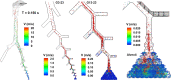
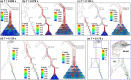
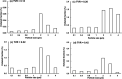
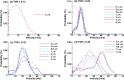
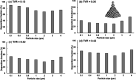
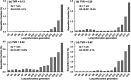
When an infected person coughs, many virus-laden droplets will be exhaled out of the mouth. Droplets from deep lungs are especially infectious because the alveoli are the major sites of coronavirus replication. However, their exhalation fraction, size distribution, and exiting speeds are unclear. This study investigated the behavior and fate of respiratory droplets (0.1-4 μm) during coughs in a single-path respiratory tract model extending from terminal alveoli to mouth opening. An experimentally measured cough waveform was used to control the alveolar wall motions and the flow boundary conditions at lung branches from G2 to G18. The mouth opening was modeled after the image of a coughing subject captured using a high-speed camera. A well-tested k-ω turbulence model and Lagrangian particle tracking algorithm were applied to simulate cough flow evolutions and droplet dynamics under four cough depths, i.e., tidal volume ratio (TVR) = 0.13, 0.20. 0.32, and 0.42. The results show that 2-μm droplets have the highest exhalation fraction, regardless of cough depths. A nonlinear relationship exists between the droplet exhalation fraction and cough depth due to a complex deposition mechanism confounded by multiscale airway passages, multiregime flows, and drastic transient flow effects. The highest exhalation fraction is 1.6% at the normal cough depth (TVR = 0.32), with a mean exiting speed of 20 m/s. The finding that most exhaled droplets from deep lungs are 2 μm highlights the need for more effective facemasks in blocking 2-μm droplets and smaller both in infectious source control and self-protection from airborne virus-laden droplets.
© 2021 Author(s).
Figures






References
-
- WHO, see https://covid19.who.int/ for “WHO Coronavirus Disease (COVID-19) Dashboard, 2020.”
-
- CDC, see https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days for “United States COVID-19 Cases and Deaths by State, 2020.”
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
