Evaluating the Impact of CYP3A5 Genotype on Post-Transplant Healthcare Resource Utilization in Pediatric Renal and Heart Transplant Recipients Receiving Tacrolimus
- PMID: 33746516
- PMCID: PMC7967030
- DOI: 10.2147/PGPM.S285444
Evaluating the Impact of CYP3A5 Genotype on Post-Transplant Healthcare Resource Utilization in Pediatric Renal and Heart Transplant Recipients Receiving Tacrolimus
Abstract
Purpose: CYP3A5 genotype is a significant contributor to inter-individual tacrolimus exposure and may impact the time required to achieve therapeutic concentrations and number of tacrolimus dose adjustments in transplant patients. Increased modifications to tacrolimus therapy may indicate a higher burden on healthcare resources. The purpose of this study was to evaluate whether CYP3A5 genotype was predictive of healthcare resource utilization in pediatric renal and heart transplant recipients.
Patients and methods: Patients <18 years of age with a renal or heart transplant between 6/1/2014-12/31/2018 and tacrolimus-based immunosuppression were included. Secondary use samples were obtained for CYP3A5 genotyping. Clinical data was retrospectively collected from the electronic medical record. Healthcare resource utilization measures included the number of dose changes, number of tacrolimus concentrations, length of stay, number of clinical encounters, and total charges within the first year post-transplant. Rejection and donor-specific antibody (DSA) formation within the first year were also collected. The impact of CYP3A5 genotype was evaluated via univariate analysis for the first year and multivariable analysis at 30, 90, 180, 270, and 365 days post-transplant.
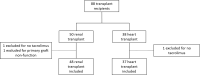
Results: Eighty-five subjects were included, 48 renal transplant recipients and 37 heart transplant recipients. CYP3A5 genotype was not associated with any outcomes in renal transplant, however, a CYP3A5 expresser phenotype was a predictor of more dose changes, more tacrolimus concentrations, longer length of stay, and higher total charges in heart transplant recipients. CYP3A5 genotype was not associated with rejection or DSA formation. Age and induction therapy were associated with higher total charges.
Conclusion: CYP3A5 genotype may predict healthcare resource utilization in the first year post-transplant, although this may be mitigated by differences in tacrolimus management. Future studies should evaluate the impact of genotype-guided dosing strategies for tacrolimus on healthcare utilization resources.
Keywords: cost of care; pediatric transplant; pharmacogenetics.
© 2021 Pasternak et al.
Conflict of interest statement
Dr Amy L Pasternak and Dr Jeong M Park report grant funding from the National Center for Advancing Translational Sciences (NCATS) to support this work under award number: UL1TR002240. The authors report no other conflicts of interest in this work.
Figures


Similar articles
-
CYP3A5 genotype affects time to therapeutic tacrolimus level in pediatric kidney transplant recipients.Pediatr Transplant. 2019 Aug;23(5):e13494. doi: 10.1111/petr.13494. Epub 2019 May 24. Pediatr Transplant. 2019. PMID: 31124575 Free PMC article.
-
Cost-effectiveness analysis of CYP3A5 genotype-guided tacrolimus dosing in solid organ transplantation using real-world data.Pharmacogenomics J. 2024 May 15;24(3):14. doi: 10.1038/s41397-024-00334-1. Pharmacogenomics J. 2024. PMID: 38750044
-
CYP3A5 polymorphisms and their effects on tacrolimus exposure in an ethnically diverse South African renal transplant population.S Afr Med J. 2020 Jan 29;110(2):159-166. doi: 10.7196/SAMJ.2020.v110i2.13969. S Afr Med J. 2020. PMID: 32657689
-
The role of pharmacogenetics in the disposition of and response to tacrolimus in solid organ transplantation.Clin Pharmacokinet. 2014 Feb;53(2):123-39. doi: 10.1007/s40262-013-0120-3. Clin Pharmacokinet. 2014. PMID: 24249597 Review.
-
CYP3A5 polymorphisms in renal transplant recipients: influence on tacrolimus treatment.Pharmgenomics Pers Med. 2018 Mar 7;11:23-33. doi: 10.2147/PGPM.S107710. eCollection 2018. Pharmgenomics Pers Med. 2018. PMID: 29563827 Free PMC article. Review.
Cited by
-
Higher number of tacrolimus dose adjustments in kidney transplant recipients who are extensive and intermediate CYP3A5 metabolizers.Clin Transplant. 2023 Apr;37(4):e14893. doi: 10.1111/ctr.14893. Epub 2023 Jan 11. Clin Transplant. 2023. PMID: 36571802 Free PMC article.
-
Tacrolimus pharmacokinetics are influenced by CYP3A5, age, and concomitant fluconazole in pediatric kidney transplant patients.Clin Transl Sci. 2023 Oct;16(10):1768-1778. doi: 10.1111/cts.13571. Epub 2023 Jun 26. Clin Transl Sci. 2023. PMID: 37340713 Free PMC article.
-
Survey of the utilization of genotype-guided tacrolimus management in United States solid organ transplant centers.Pharmacogenomics. 2025 Feb-Mar;26(3-4):89-94. doi: 10.1080/14622416.2025.2489920. Epub 2025 Apr 9. Pharmacogenomics. 2025. PMID: 40205800 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

