The Burden of Preventable Adverse Drug Events on Hospital Stay and Healthcare Costs in Japanese Pediatric Inpatients: The JADE Study
- PMID: 33746523
- PMCID: PMC7903823
- DOI: 10.1177/1179556521995833
The Burden of Preventable Adverse Drug Events on Hospital Stay and Healthcare Costs in Japanese Pediatric Inpatients: The JADE Study
Abstract
Background: Adverse drug events (ADEs) are a burden to the healthcare system. Preventable ADEs, which was ADEs due to medication errors, could be reduced if medication errors can be prevent or ameliorate.
Objective: We investigated the burden of preventable ADEs on the length of hospital stay (LOS) and costs, and estimated the national burden of preventable ADEs in pediatric inpatients in Japan.
Methods: We analyzed data from the Japan Adverse Drug Events (JADE) study on pediatric patients and estimated the incidence of preventable ADEs and associated extended LOS. Costs attributable to extended LOS by preventable ADEs were calculated using a national statistics database and we calculated the effect of preventable ADEs on national cost excess.
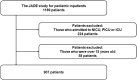
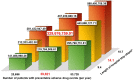
Results: We included 907 patients with 7377 patient-days. Among them, 31 patients (3.4%) experienced preventable ADEs during hospitalization. Preventable ADEs significantly increased the LOS by 14.1 days, adjusting for gender, age, ward, resident physician, surgery during hospitalization, cancer, and severe malformation at birth. The individual cost due to the extended LOS of 14.1 days was estimated as USD 8258. We calculated the annual extra expense for preventable ADEs in Japan as USD 329 676 760. Sensitivity analyses, considering the incidence of preventable ADEs and the length of hospital stay, showed that the expected range of annual extra expense for preventable ADEs in Japan is between USD 141 468 968 and 588 450 708.
Conclusion: Preventable ADEs caused longer hospitalization and considerable extra healthcare costs in pediatric inpatients. Our results would encourage further efforts to prevent and ameliorate preventable ADEs.
Keywords: Cost; Japanese pediatric inpatients; length of hospital stay; preventable ADEs; quality of healthcare.
© The Author(s) 2021.
Conflict of interest statement
Declaration of conflicting interests:The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Hitoshi Iwasaki is an employee of Novartis Pharma KK. Mio Sakuma, Hiroyuki Ida, and Takeshi Morimoto declare that there is no conflict of interest.
Figures
References
-
- Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377-384. - PubMed
-
- Bates DW, Boyle DL, Vander Vliet MB, Schneider J, Leape L. Relationship between medication errors and adverse drug events. J Gen Intern Med. 1995;10:199-205. - PubMed
-
- Laatikainen O, Miettunen J, Sneck S, Lehtiniemi H, Tenhunen O, Turpeinen M. The prevalence of medication-related adverse events in inpatients-a systematic review and meta-analysis. Eur J Clin Pharmacol. 2017;73:1539-1549. - PubMed
-
- National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP). Contemporary view of medication-related harm. A new paradigm. National Coordinating Council for Medication Error Reporting and Prevention website. 2015. Accessed November 20, 2020 https://www.nccmerp.org/sites/default/files/nccmerp_fact_sheet_2015-02-v...
LinkOut - more resources
Full Text Sources
Other Literature Sources

