Co-culture with Endothelial Progenitor Cells promotes the Osteogenesis of Bone Mesenchymal Stem Cells via the VEGF-YAP axis in high-glucose environments
- PMID: 33746579
- PMCID: PMC7976568
- DOI: 10.7150/ijms.52316
Co-culture with Endothelial Progenitor Cells promotes the Osteogenesis of Bone Mesenchymal Stem Cells via the VEGF-YAP axis in high-glucose environments
Erratum in
-
Erratum: Co-culture with Endothelial Progenitor Cells promotes the Osteogenesis of Bone Mesenchymal Stem Cells via the VEGF-YAP axis in high-glucose environments: Erratum.Int J Med Sci. 2022 Jun 12;19(6):1047-1048. doi: 10.7150/ijms.73905. eCollection 2022. Int J Med Sci. 2022. PMID: 35813290 Free PMC article.
Abstract
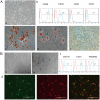
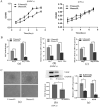
Patients with type 2 diabetes mellitus (T2DM) have a high risk of fracture and experience poor bone healing. In recent years, bone mesenchymal stem cells (BMSCs) and endothelial progenitor cells (EPCs) have become the most commonly used cells in cell therapy and tissue engineering. In this study, we found that high glucose levels had a negative effect on the differentiation of BMSCs and EPCs. Considering that EPCs-BMSCs sheets can provide endothelial cells and osteoblastic cells, we transplanted cell sheets into T2DM rats with bilateral skull defects. The outcomes of the in vivo study revealed that EPCs-BMSCs sheets promoted ossification, which was verified by micro-CT and immunohistochemistry (IHC) analyses. Furthermore, we detected the VEGF content in the culture supernatant using an enzyme-linked immunosorbent assay (ELISA). The results showed that the BMSCs co-cultured with EPCs presented a higher level of VEGF than other cells. To assess the differentiation and migration of BMSCs exposed to VEGF, ALP staining, scratch assay and qRT-PCR analysis were performed. In addition, we used immunofluorescence and western blotting analysis to further explore the related mechanisms. The results showed that cells cultured with VEGF had a stronger actin cytoskeleton and a greater amount of nuclear and total YAP than cells cultured without VEGF. Taken together, our results indicate that co-culture with EPCs could promote the osteogenesis of BMSCs partially via VEGF. Furthermore, YAP and F-actin play important roles in this process.
Keywords: BMSCs; EPCs; High glucose; VEGF; YAP; type 2 diabetes mellitus.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Liu DM, Mosialou I, Liu JM. Bone: Another potential target to treat, prevent and predict diabetes. Diabetes Obes Metab. 2018;20:1817–28. - PubMed
-
- Cancedda.R Giannoni.P, Mastrogiacomo.M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007;28:4240–50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

