Efficacy and Safety of CAR-T Therapy for Relapse or Refractory Multiple Myeloma: A systematic review and meta-analysis
- PMID: 33746596
- PMCID: PMC7976586
- DOI: 10.7150/ijms.46811
Efficacy and Safety of CAR-T Therapy for Relapse or Refractory Multiple Myeloma: A systematic review and meta-analysis
Abstract
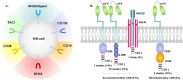
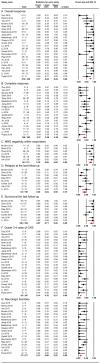
Background: Multiple myeloma (MM) is incurable in spite of recent treatment improvements, highlighting the development of new therapies. Chimeric antigen receptor (CAR) T-cell therapy has dramatically changed the therapeutic effectiveness in high-risk B-cell malignancies. For relapsed/refractory multiple myeloma (RRMM), preclinical evaluations of CAR-T therapy have shown promising efficacy, thus various active clinical trials are under way. Herein, we conducted this review to summarize efficacy and safety of CAR-T therapy and provide more evidence to guide clinical treatments. Method: We systematically searched literature based on databases (PubMed, EMBASE, Cochrane Central Register of Controlled Trials), and conference abstracts reported from American Society of Hematology (ASH), European Hematology Association (EHA) and American Society of Clinical Oncology (ASCO), in addition to other sources (www.clinicaltrials.gov, article citations). Data assessed efficacy and safety of CAR-T therapy in patients with RRMM were extracted and evaluated, and then systematically analyzed by Comprehensive Meta-analysis 3.0 (CMA 3.0). Results: A total of 23 studies including 350 participants from different countries, diagnosed as RRMM and treated with CAR-T therapy (containing 7 antigens targeted by CARs) were combined. In summary, we discovered the pooled overall response rate (77%), complete response rate (37%) and minimal residual disease (MRD) negativity rate within responders (78%). Furthermore, the pooled relapse rate of responders was 38% and median progression-free survival was 8 months. The pooled survival rate was 87% at last follow-up (median, 12 months). In addition, the pooled grade 3-4 rates of cytokine release syndrome (CRS) and neurologic toxicities (NT) were 14% and 13%, respectively. Conclusion: Our study suggests that CAR-T therapy has demonstrated efficacy and safety in RRMM patients. BCMA-targeted CAR-T and anti-BCMA contained regimen have shown better efficacy.
Keywords: BCMA; CAR-T therapy; CARs; RRMM; antigens; co-stimulatory domain; meta-analysis; systematic review.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A. 1993;90:720–4. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

