Hirsutella sinensis mycelium regulates autophagy of alveolar macrophages via TLR4/NF-κB signaling pathway
- PMID: 33746598
- PMCID: PMC7976595
- DOI: 10.7150/ijms.51654
Hirsutella sinensis mycelium regulates autophagy of alveolar macrophages via TLR4/NF-κB signaling pathway
Abstract
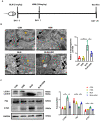
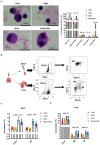
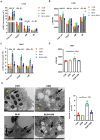
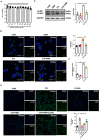
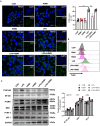
Background: Hirsutella sinensis mycelium (HSM) has potent anti-pulmonary fibrotic activities and has been proposed as an effective treatment for idiopathic pulmonary fibrosis. Macrophages are the main innate immune cells in the lung tissue, playing key roles in pulmonary fibrosis repair and homeostasis. Excessive macrophage autophagy plays a vital role in pulmonary fibrosis. The protective effect of HSM on macrophages of bleomycin (BLM)-induced pulmonary fibrotic mice remain unclear. Methods: In this study, we collected lung tissue and bronchoalveolar lavage fluid (BALF) samples from pulmonary fibrotic mice. Meanwhile, alveolar macrophages were isolated and murine macrophage RAW264.7 cell line was cultured for further study of HSM autophagy. Results: First, we found that HSM decreased the number of autophagosomes, as well as the levels of LC3B and ATG5, and increased the protein level of P62 during the development of pulmonary fibrosis. Meanwhile, HSM reduced alveolar macrophages infiltration into the BALF and inhibited their accumulation in the fibrotic lung tissue. Flow cytometry analysis showed that HSM administration inhibited the autophagy marker LC3B expression in CD11bloCD11chi alveolar macrophages in BLM-induced lung fibrosis without affecting CD11bhiCD11clo interstitial macrophages. Transmission electron microscopy and JC-1 staining for mitochondrial membrane potential of alveolar macrophages also verified that the HSM significantly decreased autophagy in the alveolar macrophages of BLM-treated mice. In vitro, autophagosomes-lysosome fusion inhibitor chloroquine (CQ) was pre-incubated with RAW264.7 cells, and HSM reduced CQ-induced autophagosomes accumulation. TLR4 signaling inhibitor CLI095 reversed the above effects, suggesting HSM could reduce the cumulation of autophagosomes dependent on TLR4. Furthermore, lipopolysaccharide (LPS)-stimulated TLR4-related autophagy was significantly inhibited by HSM treatment. In addition, the protein expressions of TLR4 and phospho-NF-κB p65 were markedly inhibited in cells treated with HSM. Conclusions: These results indicated that HSM could inhibit the autophagy of alveolar macrophages through TLR4/NF-κB signaling pathway to achieve anti-fibrotic effect.
Keywords: Hirsutella sinensis mycelium; TLR4 signal pathway.; alveolar macrophage; autophagy; pulmonary fibrosis.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
Anti-fibrosis effect for Hirsutella sinensis mycelium based on inhibition of mTOR p70S6K phosphorylation.Innate Immun. 2017 Oct;23(7):615-624. doi: 10.1177/1753425917726361. Epub 2017 Aug 24. Innate Immun. 2017. PMID: 28836874
-
[Study on treatment effect and mechanism of Hirsutella sinensis mycelium in idiopathic pulmonary fibrosis in rats].Zhongguo Zhong Yao Za Zhi. 2012 Dec;37(23):3618-23. Zhongguo Zhong Yao Za Zhi. 2012. PMID: 23477151 Chinese.
-
Iguratimod improves bleomycin-induced pulmonary inflammation and fibrosis by regulating macrophage polarization through inhibiting the TLR4/NF-κB pathway.Front Immunol. 2025 Mar 13;16:1558903. doi: 10.3389/fimmu.2025.1558903. eCollection 2025. Front Immunol. 2025. PMID: 40181990 Free PMC article.
-
Notch signaling regulates pulmonary fibrosis.Front Cell Dev Biol. 2024 Oct 10;12:1450038. doi: 10.3389/fcell.2024.1450038. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39450276 Free PMC article. Review.
-
The Role of Natural Products from Herbal Medicine in TLR4 Signaling for Colorectal Cancer Treatment.Molecules. 2024 Jun 7;29(12):2727. doi: 10.3390/molecules29122727. Molecules. 2024. PMID: 38930793 Free PMC article. Review.
Cited by
-
Qingfei Tongluo Mixture Attenuates Bleomycin-Induced Pulmonary Inflammation and Fibrosis through mTOR-Dependent Autophagy in Rats.Mediators Inflamm. 2024 Feb 8;2024:5573353. doi: 10.1155/2024/5573353. eCollection 2024. Mediators Inflamm. 2024. PMID: 38361765 Free PMC article.
-
The Role of Macrophage Autophagy in Asthma: A Novel Therapeutic Strategy.Mediators Inflamm. 2023 May 4;2023:7529685. doi: 10.1155/2023/7529685. eCollection 2023. Mediators Inflamm. 2023. PMID: 37181813 Free PMC article. Review.
-
Bushen Wenyang Huayu Decoction Targets TLR4/NF-κB Mediated Autophagy to Treat Endometriosis Effectively.Evid Based Complement Alternat Med. 2022 Nov 18;2022:4263417. doi: 10.1155/2022/4263417. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 36437825 Free PMC article.
-
Impact of Previous Pulmonary Tuberculosis on Chronic Obstructive Pulmonary Disease: Baseline Results from a Prospective Cohort Study.Comb Chem High Throughput Screen. 2023;26(1):93-102. doi: 10.2174/1386207325666220406111435. Comb Chem High Throughput Screen. 2023. PMID: 35388750 Free PMC article.
-
The Effects of the oxLDL/β2GPI/anti-β2GPI Complex on Macrophage Autophagy and its Mechanism.Immun Inflamm Dis. 2024 Nov;12(11):e70058. doi: 10.1002/iid3.70058. Immun Inflamm Dis. 2024. PMID: 39508636 Free PMC article.
References
-
- Raghu G, Rochwerg B, Zhang Y. et al. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis. An Update of the 2011 Clinical Practice Guideline. Am J Respir Crit Care Med. 2015;192:e3–19. - PubMed
-
- Samet JM, Coultas D, Raghu G. Idiopathic pulmonary fibrosis: tracking the true occurrence is challenging. Eur Respir J. 2015;46:604–6. - PubMed
-
- Navaratnam V, Fleming KM, West J. et al. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax. 2011;66:462–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

