Unraveling the Molecular Nexus between GPCRs, ERS, and EMT
- PMID: 33746610
- PMCID: PMC7943314
- DOI: 10.1155/2021/6655417
Unraveling the Molecular Nexus between GPCRs, ERS, and EMT
Abstract
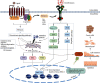
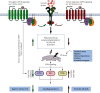
G protein-coupled receptors (GPCRs) represent a large family of transmembrane proteins that transduce an external stimulus into a variety of cellular responses. They play a critical role in various pathological conditions in humans, including cancer, by regulating a number of key processes involved in tumor formation and progression. The epithelial-mesenchymal transition (EMT) is a fundamental process in promoting cancer cell invasion and tumor dissemination leading to metastasis, an often intractable state of the disease. Uncontrolled proliferation and persistent metabolism of cancer cells also induce oxidative stress, hypoxia, and depletion of growth factors and nutrients. These disturbances lead to the accumulation of misfolded proteins in the endoplasmic reticulum (ER) and induce a cellular condition called ER stress (ERS) which is counteracted by activation of the unfolded protein response (UPR). Many GPCRs modulate ERS and UPR signaling via ERS sensors, IRE1α, PERK, and ATF6, to support cancer cell survival and inhibit cell death. By regulating downstream signaling pathways such as NF-κB, MAPK/ERK, PI3K/AKT, TGF-β, and Wnt/β-catenin, GPCRs also upregulate mesenchymal transcription factors including Snail, ZEB, and Twist superfamilies which regulate cell polarity, cytoskeleton remodeling, migration, and invasion. Likewise, ERS-induced UPR upregulates gene transcription and expression of proteins related to EMT enhancing tumor aggressiveness. Though GPCRs are attractive therapeutic targets in cancer biology, much less is known about their roles in regulating ERS and EMT. Here, we will discuss the interplay in GPCR-ERS linked to the EMT process of cancer cells, with a particular focus on oncogenes and molecular signaling pathways.
Copyright © 2021 Niti Kumari et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures

Similar articles
-
Calreticulin promotes EMT in pancreatic cancer via mediating Ca2+ dependent acute and chronic endoplasmic reticulum stress.J Exp Clin Cancer Res. 2020 Oct 7;39(1):209. doi: 10.1186/s13046-020-01702-y. J Exp Clin Cancer Res. 2020. PMID: 33028359 Free PMC article.
-
HCV induces transforming growth factor β1 through activation of endoplasmic reticulum stress and the unfolded protein response.Sci Rep. 2016 Mar 1;6:22487. doi: 10.1038/srep22487. Sci Rep. 2016. PMID: 26927933 Free PMC article.
-
PARP16 is a tail-anchored endoplasmic reticulum protein required for the PERK- and IRE1α-mediated unfolded protein response.Nat Cell Biol. 2012 Nov;14(11):1223-30. doi: 10.1038/ncb2593. Epub 2012 Oct 28. Nat Cell Biol. 2012. PMID: 23103912 Free PMC article.
-
The multiple roles of the unfolded protein response regulator IRE1α in cancer.Mol Carcinog. 2019 Sep;58(9):1623-1630. doi: 10.1002/mc.23031. Epub 2019 Apr 30. Mol Carcinog. 2019. PMID: 31041814 Free PMC article. Review.
-
Emerging roles for the ER stress sensor IRE1α in metabolic regulation and disease.J Biol Chem. 2019 Dec 6;294(49):18726-18741. doi: 10.1074/jbc.REV119.007036. Epub 2019 Oct 30. J Biol Chem. 2019. PMID: 31666338 Free PMC article. Review.
Cited by
-
Unraveling the unfolded protein response signature: implications for tumor immune microenvironment heterogeneity and clinical prognosis in stomach cancer.Aging (Albany NY). 2024 May 2;16(9):7818-7844. doi: 10.18632/aging.205784. Epub 2024 May 2. Aging (Albany NY). 2024. PMID: 38700505 Free PMC article.
-
Synergistic anticancer activity of cisplatin combined with tannic acid enhances apoptosis in lung cancer through the PERK-ATF4 pathway.Eur J Med Res. 2023 Oct 27;28(1):462. doi: 10.1186/s40001-023-01420-z. Eur J Med Res. 2023. PMID: 37885044 Free PMC article.
-
FXR1 promotes proliferation, invasion and migration of hepatocellular carcinoma in vitro and in vivo.Oncol Lett. 2022 Nov 22;25(1):22. doi: 10.3892/ol.2022.13608. eCollection 2023 Jan. Oncol Lett. 2022. PMID: 36466996 Free PMC article.
-
G-protein-coupled receptor 141 mediates breast cancer proliferation and metastasis by regulating oncogenic mediators and the p-mTOR/p53 axis.Oncotarget. 2023 May 19;14:466-480. doi: 10.18632/oncotarget.28433. Oncotarget. 2023. PMID: 37204251 Free PMC article.
-
Elucidation of anti-human melanoma and anti-aging mechanisms of compounds from green seaweed Caulerpa racemosa.Sci Rep. 2024 Nov 11;14(1):27534. doi: 10.1038/s41598-024-78464-6. Sci Rep. 2024. PMID: 39528552 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

