The impact of publicly funded rotavirus immunization programs on Canadian children
- PMID: 33746618
- PMCID: PMC7968475
- DOI: 10.14745/ccdr.v47i02a02
The impact of publicly funded rotavirus immunization programs on Canadian children
Abstract
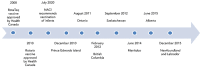
Background: In 2008, the National Advisory Committee on Immunization recommended routine rotavirus immunizations in healthy Canadian infants. Over the following seven years, eight provinces and two territories introduced the rotavirus vaccine into their publicly funded immunization programs.
Objective: Assess the burden of rotavirus infections before and after implementation of publicly funded immunization programs.
Methods: We analyzed laboratory-confirmed community cases of rotavirus reported to the National Enteric Surveillance Program and hospitalizations of children younger than three years old from 2007 to 2017 with rotavirus diagnosis-specific ICD-10 codes. Rates of illness were calculated for each province for the two years prior to and after implementation of public funding of the vaccine. The year of implementation was not included to accommodate the uptake period of the vaccine. Age-specific rates were assessed in jurisdictions where five years of data were available the year after the vaccine was publicly funded. The pre-post and difference-in-difference (DID) methodologies were applied to hospital discharge data to evaluate changes between the funding and non-funding jurisdictions.
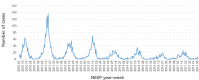
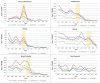
Results: Community cases of laboratory-confirmed rotavirus infection reported to the National Enteric Surveillance Program declined by 54% between 2010 and 2017. Rates of hospital discharges decreased significantly among children in six provinces after the adoption of the rotavirus vaccine. Hospital discharge rates in Alberta, Manitoba, Ontario and Prince Edward Island dropped between 53% and 71%, and by 75% for British Columbia and Saskatchewan.
Conclusion: Public funding of the rotavirus vaccine appeared to lead to significant reductions in laboratory-confirmed rotavirus cases reported to the National Enteric Surveillance Program and in the rates of rotavirus gastroenteritis-related hospital discharges.
Keywords: burden; evaluation; intervention; rotavirus; vaccination.
Conflict of interest statement
Competing interests: None.
Figures
Similar articles
-
Knowledge, attitudes, beliefs, and behaviors of parents and healthcare providers before and after implementation of a universal rotavirus vaccination program.Vaccine. 2016 Jan 27;34(5):687-695. doi: 10.1016/j.vaccine.2015.09.089. Epub 2015 Oct 14. Vaccine. 2016. PMID: 26458809
-
Equity and impact: Ontario's infant rotavirus immunization program five years following implementation. A population-based cohort study.Vaccine. 2019 Apr 17;37(17):2408-2414. doi: 10.1016/j.vaccine.2019.01.061. Epub 2019 Feb 11. Vaccine. 2019. PMID: 30765171
-
A population based study comparing changes in rotavirus burden on the Island of Ireland between a highly vaccinated population and an unvaccinated population.Vaccine. 2016 Sep 7;34(39):4718-4723. doi: 10.1016/j.vaccine.2016.08.004. Epub 2016 Aug 12. Vaccine. 2016. PMID: 27527819
-
Update on Rotarix: an oral human rotavirus vaccine.Expert Rev Vaccines. 2009 Dec;8(12):1627-41. doi: 10.1586/erv.09.136. Expert Rev Vaccines. 2009. PMID: 19943758 Review.
-
Uptake, impact, and effectiveness of rotavirus vaccination in the United States: review of the first 3 years of postlicensure data.Pediatr Infect Dis J. 2011 Jan;30(1 Suppl):S56-60. doi: 10.1097/INF.0b013e3181fefdc0. Pediatr Infect Dis J. 2011. PMID: 21183842 Review.
Cited by
-
The success of publicly funded rotavirus vaccine programs for preventing community- and hospital-acquired rotavirus infections in Canadian pediatric hospitals: an observational study.CMAJ Open. 2023 Dec 19;11(6):E1156-E1163. doi: 10.9778/cmajo.20220245. Print 2023 Nov-Dec. CMAJ Open. 2023. PMID: 38114258 Free PMC article.
-
Clinical Profiles of Childhood Astrovirus-, Sapovirus-, and Norovirus-Associated Acute Gastroenteritis in Pediatric Emergency Departments in Alberta, 2014-2018.J Infect Dis. 2022 Feb 15;225(4):723-732. doi: 10.1093/infdis/jiab429. J Infect Dis. 2022. PMID: 34432027 Free PMC article.
References
-
- Health Canada. Notice of compliance information. Ottawa (ON): Health Canada; (modified 2019-10-21; accessed 2020-05-05). https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailOne.php...
-
- Health Canada. Notice of compliance database search results. Ottawa (ON): Health Canada; (modified 2019-10-21; accessed 2020-05-05). https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailOne.php...
-
- An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI). Statement on the recommended use of pentavalent human-bovine reassortant rotavirus vaccine. Can Commun Dis Rep 2008;34(ACS-1):1–33. https://www.canada.ca/en/public-health/services/reports-publications/can... - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources