Quantitative investigation of the urinary excretion of three specific monoester metabolites of the plasticizer diisononyl adipate (DINA)
- PMID: 33746670
- PMCID: PMC7975632
- DOI: 10.17179/excli2021-3360
Quantitative investigation of the urinary excretion of three specific monoester metabolites of the plasticizer diisononyl adipate (DINA)
Abstract
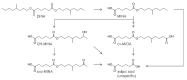
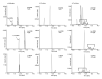
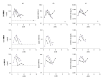
Diisononyl adipate (DINA) is a plasticizer used in PVC products as an alternative for restricted phthalate plasticizers. With this study, we provide first data on human DINA metabolism and excretion. We postulated mono(hydroxy-isononyl) adipate (OH-MINA), mono(oxo-isononyl) adipate (oxo-MINA), and mono(carboxy-isooctyl) adipate (cx-MIOA) as specific DINA metabolites based on the known human metabolism of structurally similar adipates and phthalates. Urinary excretion was quantitatively investigated after a single oral dose (113 to 145 µg/kg body weight) to three healthy volunteers using a newly developed online-SPE-LC-MS/MS method with isotope dilution and LOQs between 0.3 - 0.6 µg/L. OH-MINA turned out to be the major of the three metabolites with consistent urinary excretion fractions (FUEs) of 0.020-0.023 % among all volunteers. Oxo-MINA and cx-MIOA were excreted with lower shares (mean: 0.003 % and 0.009 %, respectively). For all three metabolites, urinary concentrations peaked quickly between 1.4 and 2.3 h post dose with maximum concentrations of 23.1 (OH-MINA), 2.87 (oxo-MINA) and 9.83 µg/L (cx-MIOA). Thus, FUEs and urinary concentrations were rather low for these specific metabolites, with the major share of the dose presumably being excreted as non-specific metabolites such as adipic acid. In a pilot population (n=35) of German adults without known DINA exposure, we could not detect any of the three metabolites, contrary to the dosage study, indicating to population exposures lower than 50 µg/kg body weight/day. The new HBM method in conjunction with the new FUEs can be used for objective DINA exposure and risk assessment especially in populations with potentially higher DINA exposures.
Keywords: DINA; diisononyl adipate; human biomonitoring; metabolism; oral dose; plasticizer.
Copyright © 2021 Gotthardt et al.
Figures


Similar articles
-
Human metabolism and urinary excretion kinetics of di-n-butyl adipate (DnBA) after oral and dermal administration in three volunteers.Toxicol Lett. 2021 Jun 1;343:11-20. doi: 10.1016/j.toxlet.2021.02.012. Epub 2021 Feb 25. Toxicol Lett. 2021. PMID: 33640488 Clinical Trial.
-
Metabolism and urinary excretion kinetics of di(2-ethylhexyl) adipate (DEHA) in four human volunteers after a single oral dose.Toxicol Lett. 2020 Mar 15;321:95-102. doi: 10.1016/j.toxlet.2019.12.006. Epub 2019 Dec 6. Toxicol Lett. 2020. PMID: 31816331
-
Determination of di-n-butyl adipate (DnBA) metabolites as possible biomarkers of exposure in human urine by online-SPE-LC-MS/MS.J Chromatogr B Analyt Technol Biomed Life Sci. 2020 Mar 15;1141:122029. doi: 10.1016/j.jchromb.2020.122029. Epub 2020 Feb 13. J Chromatogr B Analyt Technol Biomed Life Sci. 2020. PMID: 32109750
-
[Toxicity effects of phthalate substitute plasticizers used in toys].Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 2012;(130):31-42. Kokuritsu Iyakuhin Shokuhin Eisei Kenkyusho Hokoku. 2012. PMID: 23243985 Review. Japanese.
-
Phthalates: metabolism and exposure.Int J Androl. 2008 Apr;31(2):131-8. doi: 10.1111/j.1365-2605.2007.00837.x. Epub 2007 Dec 7. Int J Androl. 2008. PMID: 18070048 Review.
Cited by
-
Global Environmental and Toxicological Data of Emerging Plasticizers: Current Knowledge, Regrettable Substitution Dilemma, Green Solution and Future Perspectives.Green Chem. 2024 May 21;26(10):5635-5683. doi: 10.1039/d3gc03428c. Epub 2024 Apr 16. Green Chem. 2024. PMID: 39553194 Free PMC article.
-
Human metabolism and urinary excretion of seven neonicotinoids and neonicotinoid-like compounds after controlled oral dosages.Arch Toxicol. 2022 Jan;96(1):121-134. doi: 10.1007/s00204-021-03159-0. Epub 2021 Oct 13. Arch Toxicol. 2022. PMID: 34642770 Free PMC article.
References
-
- Abe Y, Sugita T, Wakui C, Niino T, Yomota C, Ishiwata H, et al. Material labeling of soft plastic toys and plasticizers in polyvinyl chloride products (Paper in Japanese, abstract and tables in English) Shokuhin Eiseigaku Zasshi. 2003;44:168–74. - PubMed
-
- Abe Y, Yamaguchi M, Mutsuga M, Hirahara Y, Kawamura Y. Survey of plasticizers in polyvinyl chloride toys (Paper in Japanese, abstract and tables in English) Shokuhin Eiseigaku Zasshi. 2012;53:19–27. - PubMed
-
- Anderson WAC, Castle L, Hird S, Jeffery J, Scotter MJ. A twenty-volunteer study using deuterium labelling to determine the kinetics and fractional excretion of primary and secondary urinary metabolites of di-2-ethylhexylphthalate and di-iso-nonylphthalate. Food Chem Toxicol. 2011;49:2022–9. - PubMed
-
- Angerer J, Ewers U, Wilhelm M. Human biomonitoring: State of the art. Int J Hyg Environ Health. 2007;210:201–28. - PubMed
-
- Apel P, Kortenkamp A, Koch HM, Vogel N, Rüther M, Kasper-Sonnenberg M, et al. Time course of phthalate cumulative risks to male developmental health over a 27-year period: Biomonitoring samples of the German Environmental Specimen Bank. Environ Int. 2020;137:105467. - PubMed
LinkOut - more resources
Full Text Sources
